
Coronavirus
Technology Solutions
October 27, 2021
Intertek is Evaluating Efficacy of Masks After
Long Shelf Life
Intertek Provides Mask Labels
Fit Testing Clinics for School Teachers in N95
Masks in Grants Pass, Oregon
Eugene Fire Dept Requires Fit Tested N95 Masks
for the Unvaccinated
Consumer Reports Emphasizes the Importance of
Tight Fit
Australian City Bans Beards and Requires Tight
Fitting Masks
MAG HEPA-Grade Meltblown Targets Medical
Industry
Meltblown Nonwoven Sales to Double to $1.68
billion Due to Covid Facemask and Medical
Disposables Surge
Daikin Strategy Based on Fusion
MANN+HUMMEL Cabin Air Filter Achieves CN95
Efficiency
______________________________________________________________________________
Intertek is Evaluating Efficacy of Masks After
Long Shelf Life
Last year Intertek, won a multi-year contract
with the U.S. National Institute for
Occupational Safety and Health (NIOSH) for
testing post-market N95 respirators. The
three-year contract expands on NIOSH’s existing
agreement with Intertek to conduct
precertification evaluations on new respirators.
The Company will now assess respirators on the
market that have exceeded their original shelf
life; respirators entering the United States
from a foreign country and claiming to meet
non-NIOSH standards; and previously used
respirators that have been decontaminated and
are entering the market again.
N95 respirators reduce a wearer’s exposure to
particles, filtering out at least 95 per cent of
very small particles, including bacteria and
viruses. They must be evaluated and approved by
NIOSH according to requirements set forth in
NIOSH 42 CFR Part 84. Under this regulation,
manufacturers must submit data which illustrates
compliance, including precertification tests
such as inhalation/exhalation resistance,
particulate filtration efficiency, and valve
leakage. Intertek will conduct the
precertification testing on post-market
respirators, providing manufacturers with the
necessary data for their NIOSH submission and
approval.
Jason Allen, Technical Lead at Intertek said,
“Intertek’s purpose is to bring quality and
safety to life and ensuring the quality and
safety of N95 respirators has never been more
important as the world continues to combat the
COVID-19 pandemic. We are thrilled to use our
experience in testing N95 respirators and other
personal protective equipment to help NIOSH and
respirator manufacturers get these essential and
potentially life-saving products to market.”
Intertek provides rigorous evaluations and
expansive testing capabilities to deliver
assurance that, even under the most stressful or
hazardous conditions, personal protective
equipment will provide sustained protection. The
company’s industry-leading PPE testing services
yield third-party test reports accepted by the
Safety Equipment Institute (SEI) for a wide
array of standards, assuring customers that a
product meets performance and industry
requirements.
Intertek Provides Mask Labels
Intertek has developed an innovative Mask Label
Program to support their customers in
communicating the verified quality and
performance attributes of mask products,
including medical masks, respirators and
community masks, to their stakeholders through a
trusted tool. The Mask Label Program also helps
to promote quality and increase traceability of
mask products on the market. The Mask Label
Program is a voluntary program free of charge
for Intertek customers who have tested their
mask products at our PPE Centres of Excellence (CoE)
and fulfilled all mandatory test or recommended
requirements of international or national
standards or guidance.
Under the program, a mask label showing the
testing standard or the guidance and the
classification attained (if any), together with
a unique QR code that links to the mask
information on the Intertek PPE
CoE Directory,
will be granted to successful applicants with an
aim to help them promote the visibility of
tested performance of their mask products
through third party verification.
Here are the
types of mask labels that the program offers.

From left to right: medical mask, respirator,
community mask
Benefits
-
A trusted tool to showcase
verified quality and performance
attributes of your mask products
to give peace of mind to
consumers
-
Differentiate your quality
products from other masks in the
market through third-party
testing and verification
-
Instant access to verified mask
information with a scan of QR
code
-
Increase traceability of your
mask products
-
Increase business opportunities
by listing your products in the
Intertek PPE CoE Directory
The Intertek PPE Centres of Excellence offer
Total Quality Assurance solutions to support the
sourcing, procurement, manufacturing and R&D
activities for masks.
Fit Testing Clinics for School Teachers in N95
Masks in Grants Pass, Oregon
Grants Pass School District 7
is requiring that unvaccinated staff wear N95
masks. The district will supply the masks and is
working with the county’s public health to
provide fit-testing clinics.
Weekly testing is not part of
that protocol, though the president of the
Grants Pass teachers union says that was brought
up in discussions.
“I think, ideally, we all
think there should be some kind of cross section
testing for everybody so we always have a pretty
good idea of where we are with positive cases.
But we’re a school, not a health care facility,”
Mickey Laney-Jarvis explained. She is with
Grants Pass Education Association.
Jarvis says that about 80% of
district employees are either vaccinated or will
be vaccinated by the deadline and 17% are
seeking an exception. That leaves about 3%that
have decided to either take leave, or no longer
be employed by the district.
Eugene Fire Dept
Requires Fit Tested N95 Masks for the
Unvaccinated
Eugene Oregon Fire department requires
unvaccinated employees to wear fit tested N95
masks.
According to Eugene Springfield Fire, 92.6% of
its staff members are fully vaccinated. That
accounts for 291 of the department's 314 total
employees. Forty-eight staff members are
non-licensed healthcare providers, which means
the mandate does not apply to them.
Of the staff members who are licensed healthcare
providers, 21 employees have asked for medical
or religious exemptions and two have not
requested an exemption.
The department said it has accommodated its
unvaccinated staff members by requiring them to
wear fit-tested N95 masks at all times,
disinfect their private dorm at the end of their
shift, and remain at least six feet apart from
other staff members when eating or drinking.
Unvaccinated staff members will have to follow
routine testing requirements if their lead
agencies or the city of their employment
implements them, Eugene Springfield Fire said.
Consumer Reports
Emphasizes the Importance of Tight Fit
The N95 is intended as a workplace mask, so the
NIOSH standards are meant to ensure it provides
adequate protection on the job. To maintain that
certification, regular quality control is
required, explains Anne Miller, executive
director of Project N95, a nonprofit
organization that sources high-quality and
reliable masks and other personal protective
equipment (PPE) for healthcare workers and the
general public.
Although many cloth masks may not perform as
well as medical masks, upgrading your mask is
about more than just the material it’s made out
of. A well-fitting cloth mask made from at least
three layers of tightly woven cloth (with the
middle layer being a different type of fabric),
would probably outperform a surgical mask that
has gaps on the sides and is constantly sliding
down off your nose says. Luis
Jimenez, PhD, an aerosol scientist at the
University of Colorado Boulder.
That’s because air takes the path of least
resistance. “Any gap between the face and the
mask defeats the purpose, because the air has a
much easier time going through those gaps
instead of going through the cloth of the mask,”
according to Jimenez. This is a potential
problem even with highly effective but
ill-fitting N95, KN95, and KF94 respirators.
There are a number of strategies you can use to
get a better fit, such as double masking, using
a mask brace or fitter, or strategically
knotting ear loops.
Different face shapes and sizes may be suited to
different types of masks. If you buy one type of
mask and find it doesn’t fit and that you can’t
adapt it well enough to close the gaps, don’t
give up. Try another brand or style.
Consumers Report says it’s
a good idea to look for ASTM standards not just
when you’re buying a cloth mask but also when
you’re buying a surgical mask. Surgical mask
makers can make products that meet ASTM
standards for the material used, which
guarantees certain levels of filtration and
breathability. (These are different standards
from those used to evaluate cloth and nonmedical
masks.) Look for masks that are labeled ASTM
Level 1, 2, or 3. You’ll still need to make sure
the mask fits well to your face, however,
because the certification applies only to the
mask’s filter material and not its overall
performance.
And it can be tricky to make sure you’re not
getting a counterfeit KN95 or equivalent face
covering. But a few reliable sources exist,
including this list
of products tested by the National Personal
Protective Technology Laboratory. Project
N95 is another resource. And although
the FDA revoked its blanket Emergency Use
Authorizations (EUA) for various types of
personal protective equipment earlier this year,
the list
of products that received the EUA is
also a good place to look, Miller says.
Australian City Bans
Beards and Requires Tight Fitting Masks
A major Melbourne health organization is
implementing a beard ban.
Western Health says it is not possible to
achieve a good seal of an N95 mask with a beard,
so workers must shave for fit testing.
If staff decline to shave they will be
redeployed.
In a statement, Western Health said “Staff
safety is our highest priority and our policies
on PPE are based around that. It is not possible
to achieve a good seal of an N95 mask with a
beard
MAG HEPA-Grade Meltblown Targets Medical
Industry
Magical Film Enterprise (MAG) is the largest
manufacturer of nonwoven fabrics in Taiwan and
has the capacity of over 100 tons per month for
the nonwoven products. All products are
certified by Nelson Labs, FDA, SGS, TTRI,
ROHS&REACH. For quality control, MAG uses
TSI-8130 to test each roll when producing. This
automated filter tester is often used by
international testing lab, such as Nelson Labs
and TTRI.
MAG manufactures both hydrophobic and
hydrophilic nonwoven fabric. Both are made by
using PP material. MAG spunbond nonwoven can be
applied on medical material like surgical masks
and scrubs. The nonwoven provides high
customization for customers, particularly in
color. Beside traditional green and blue, MAG
also has Primrose yellow, Tulip yellow, Light
lilac, Baby blue eyes, Sky blue, Mint green,
Angel white, Black, etc. Any color can be tailor
made for customers.
As for meltblown, MAG launched HEPA grade
meltblown for the beauty, medical and personal
protective filter material industry. By using
the water electret technique, it provides higher
efficiency and higher permeability. MAG HEPA can
meet the standard from H10-H14. Comparing with
the general meltblown, HEPA grade has lower air
resistance while maintaining higher performance.
HEPA can effectively remove at least 99% of
aerosols 0.3 micrometers (μm) in diameter, also
prevent and remove the spread of dust, pollen,
and PM 2.5.
MAG was established in 2001 and founded in
Taiwan. In 2020, it was awarded the ambassador
of Taiwan National Team for Face Mask and D&B
TOP 1000 Elite SME.
Meltblown Nonwoven Sales to Double to $1.68
billion Due to Covid Facemask and Medical
Disposables Surge
The global market for meltblown nonwovens has
been fundamentally reshaped by the experience of
Covid-19. Demand for vital products like face
masks and medical PPE saw meltblown sale rises
from $809 million in 2019 to $1.68 billion in
2020.
As this process has become a primary concern for
nonwovens producers and converters it is
profiled in depth in a new dedicated Smithers study
– The Future of Meltblown Nonwovens to 2026.. It
gives provides detailed market data for
meltblowns (by tonnage, surface area, raw
material cost, and sales value) charting the
radical changes that have characterized the
market since the beginning of 2020.
In the first days of the pandemic professional
and medical-grade PPE – especially N95 medical
face masks – became a vital commodity. Smithers’
data show how demand increased nearly 10-fold
from 14,400 tons in 2019 to 121,800 tons in
2020, while other medical meltblown applications
rose five-fold. This led to meltblown production
sites running at near or overcapacity; and other
production facilities, like SMS, being switched
over to meltblown manufacture. A host of new
lines have been commissioned worldwide to raise
capacity and ensure domestic availability.
The reduction of the Covid threat through H2
2021 is leading to a slight fall in the market
for 2021 after this unprecedented peak. Residual
fears over Covid and the need to establish
strategic stockpiles against similar outbreaks
in the future mean that demand will remain well
above pre-pandemic levels through to 2026. A
total of 302,700 tons, or 5.07 billion square
meters, with a sales value of $1.17 billion will
be sold in that year.
The experience has brought a major – if
temporary – shift in the meltblown markets. The
importance of face mask media has led to the
fine fiber meltblowns (4 micron or less)
overtaking coarser standard fiber meltblowns
(4-15 microns). Simultaneously the rise in
demand for disposable products have seen these
overtake durable meltblown nonwovens as the main
market application – disposables’ share rose
from 40% in 2019, to 64.6% of total consumption
in 2020. Though a return of the pre-Covid mix of
durables and disposables is forecast by 2026.
For many durable meltblown the closure of
end-use industries like construction and
automotive production, impacted sales. While
some lines were repurposed in the short term the
market will benefit in the future from new
infrastructure spending plans and the desire for
improve fine mesh filtration media.
New meltblown production lines have been built
and entered operation in record time across the
pandemic period. As demand for facemasks
slackens in subsequent years the market will
face an oversupply of fine fiber meltblown
capacity – as much as 77% of this new is
untenable in the longer term. This will lead to
lower prices if polypropylene prices stabilize,
and the closure of some older lines. Government
support to protect local meltblown production
and converting capacity for PPE as a strategic
resource will not eliminate the imperative to
develop new end-use applications for meltblown
output.
One other factor that will emerge over the
five-year Smithers forecast period is the need
for greater sustainability in nonwovens.
Performance requirements mean meltblowns will
continue to rely almost exclusively on plastic
feedstocks, especially polypropylene. It
represents 91% of meltblown materials in 2021,
with only minor shares for other polymer types.
Across 2021-2026 there is potential to integrate
bio-based polymers, including some more
biodegradable grades. Other advances in waste
reduction, lower basis weight materials, and use
of recycled polymers are also options. For
meltblown nonwovens these are not anticipated to
have a significant impact before 2026. These
products may ultimately benefit from
technologies developed for other nonwoven
processes, but meltblown will continue to be a
segment defined by performance.
The impact of the pandemic, and the future shape
of demand in this dynamic nonwoven sector is
examined in forensic detail in the new Smithers
study The Future of Meltblown Nonwovens to 2026.
Historic, current and future demand is
quantified with a high degree of granularity and
segmented by raw material.
Daikin Strategy Based on Fusion
Daikin defines the Meaning of the Term Fusion as
- Fuse together various business aspects -
-
Both short-term profitability
and long-term growth
-
Collaborations among Group
companies worldwide
-
Partnerships with non-Group
companies Etc.
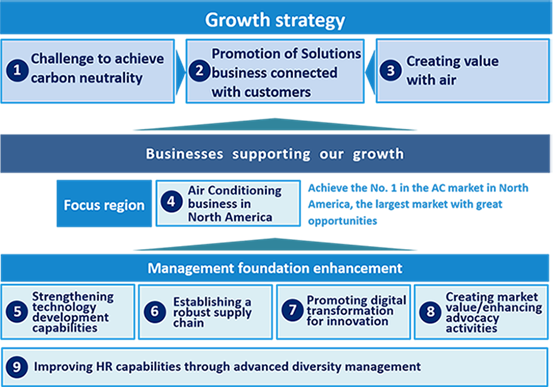
One
initiative involves greenhouse gas reduction
through heat recovery. The Daikin HRV (Heat
Reclaim Ventilation) recovers heat energy lost
through ventilation and holds down temperature
changes caused by ventilation, thereby
maintaining a comfortable and clean environment.
This also reduces the load on the air
conditioning system and conserves energy.
In addition, the HRV interlocks with Daikin’s
VRV system, Sky Air and other air conditioning
systems and automatically switches over
ventilation mode, further increasing the effects
of energy conservation. HRV operation has been
centralized on the air conditioner remote
control allowing total control over air
conditioning and ventilation via a simple
configuration. The current line-up includes
models with DX coil and/or humidifier - the DX
coil helps prevent the direct impact of cold
airflow upon personnel during the heating cycle
and vice versa.
MANN+HUMMEL Cabin Air Filter Achieves CN95
Efficiency
In February 2020, the China Automotive
Technology & Research Center Co. Ltd. launched
the CN95 certification. This is based on test
standards previously developed by the research
institute for its market study on the Chinese
cabin air filter market.
The CN95 certification is setting new standards
in the cabin air filter market, thus striving to
improve the quality level of cabin air filters,
which is quite low among independent aftermarket
products. It is, however, not yet a mandatory
requirement for the sales of cabin air filters
in China. As awareness of air pollution and its
impact on health, as well as concerns about
viruses, continue to grow, certification could
have a positive impact on consumer perception in
the future.
The main requirements for certification are
pressure drop, dust holding capacity and
fractional efficiency. In the meantime limits
were slightly modified for the additional
certification of odor and gas adsorption. To
reach the upper CN95 efficiency level (TYPE I),
the media used in the cabin filter needs to
filter out more than 95 percent of particles
with a diameter larger than 0,3 µm. This means
that fine dust particles, bacteria and virus
aerosols can be blocked. Fine dust particles are
a serious health concern because they can pass
through the lungs and into the bloodstream,
causing serious illness.

Since early 2020 MANN+HUMMEL has been supporting
OE customers successfully on the CN95
certification which can be only applied for at
CATARC’ s subsidiary “CATARC Huacheng
certification Co., Ltd. in Tianjin. With its
wide portfolio of filter media, MANN+HUMMEL can
upgrade the filtration efficiency of cabin air
filters in the original equipment and in the
aftermarket. The cabin filter range includes
particle filters and combi filters with
activated carbon (for the adsorption of gases
and odors). For an additional inactivation of
microorganisms like allergens or bacteria a bio
functional layer needs to be added to the
activated carbon media. Optionally, a further
nanofiber layer provides a high efficient fine
dust particle filtration over the whole filter
lifetime.
This combination of layers and media enables
MANN+HUMMEL not only to meet the requirements of
CN95 certification, but also to offer people in
the vehicle the best possible protection against
air pollution.
