
Coronavirus
Technology Solutions
March 16, 2021
How Large will
the Mask Market
be in 2022 and
Beyond?
OSHA Mask
Standard has
been Delayed
OSHA has the
Power to Set and
Enforce Mask
Standards
OSHA Could
Require Masks to
Meet the
ASTM
Standard
Mask
Manufacturers
Cite Present
Production of
One Billion High
Efficiency Masks
per Month
Italy Institutes
Lockdown with
Other EU
Countries Also
Pulling Back
Three EU
Countries
Suspend
AstraZeneca
Vaccine Use
Halo Life Mask
Meets ASTM
Standard
____________________________________________________________________________
How Large will
the Mask Market
be in 2022 and
Beyond?
With high
efficiency mask
mandates likely
in much of the
world there will
be a huge market
for public masks
over the next
year. Mask
suppliers are
debating how
much effort to
place on this
opportunity. The
question is
whether the
market could
shrink back to
the $500 million
market of 2019.
First of all it
is likely that
the COVID market
will remain at
some level for
years to come.
It will be
several years
before the world
reaches herd
immunity.
Vaccines may be
only effective
for one year.
New variants
could cause
trouble for 10
years according
to some
predictions.
So the COVID
segment of the
market could
drop from $80
billion in 2021
to $40 billion
in 2022 and $20
billion in 2023.
What about the
traditional
market which
includes
courtesy masks,
air pollution
protection, dust
storms,
wildfires, Red
Tide, and other
infectious
diseases? WHO
says eight
million people
are dying
because of air
pollution each
year. The
dangers of
smoking were
known for years
before there was
eventually
guidance and
regulation.
Courtesy masks
alone could be a
very big market.
The average
person has two
colds per year.
So he
might be
infectious for
1/25 of the
year. It is very
likely that the
Asian “courtesy”
mask custom will
become
institutionalized.
OSHA could alter
the temporary
mask policy to
reduce the
general
requirement but
leave it in
place for anyone
showing signs of
a cold or flu.
If you are a
restaurant owner
and have to
implement mask
wearing for
infectious
servers that
will provide a
negative image.
On the other
hand if you
require all
servers to wear
masks it creates
a positive
image.
To no small
extent the
future use of
masks will be a
function of
CATER: Comfort,
Attractiveness,
Tight fit,
Efficiency,
Reusability or
life
OSHA Mask
Standard has
been Delayed
The White
House's
self-imposed
deadline to
impose a
nationwide face
mask standard in
workplaces won't
be met, since
deliberations
are still
underway.
President Biden
on January 21
directed the
Labor
Department's
Occupational
Safety and
Health
Administration
to determine by
Monday, March
15, if such a
standard is
needed.
An emergency
temporary
standard on face
masks in the
workplace is
ultimately
expected to be
issued, but the
analysis has not
yet been
completed, three
people familiar
with the process
told CBS News.
The nationwide
temporary
standard for
face masks in
the workplace
would impact
millions of
workers and
would last six
months.
Public health
and workplace
safety experts
told CBS News
the emergency
temporary
standard could
provide valuable
social
distancing
advice for
workers and
safety guidance
for face masks,
since they vary
in their
protective
abilities.
OSHA workplace
standards are
enforceable and
usually take
years to
implement, so an
emergency
temporary
standard has
occasionally
been used to
avoid normal
rulemaking
procedures so
that "grave
dangers" facing
workers can be
addressed.
The last time
the emergency
temporary
standards
process was used
was in 1983 to
limit asbestos
exposure in the
workplace,
according to
the Congressional
Research
Service. The
rule was
eventually
struck down in
court. The
nationwide
temporary
standard for
face masks in
the workplace
would impact
millions of
workers and
would last six
months. In its
absence, several
states have
enacted their
own standards.
Without a
nationwide
standard so far
during the
pandemic,
several states
have enacted
their own
standards.
Dr. David
Michaels, a
former assistant
secretary of
labor for OSHA
who served on
Mr. Biden's
presidential
transition team,
explained to CBS
News the
technical
aspects of any
emergency
temporary
standard must be
balanced with
the urgent need
to protect
workers from the
spread of
COVID-19.
"OSHA lost a
year because the
Trump
administration
refused to
prepare an
emergency
standard," Dr.
Michaels said,
"The longer the
delay, the more
exposures will
occur."
"OSHA has to
show that the
precautions
required by the
standard are
effective in
preventing
exposure while
at the same time
being
economically and
technically
feasible for
employers," Dr.
Michaels added.
"The
Occupational
Safety and
Health
Administration
has been working
diligently, as
appropriate, to
consider what
standards may be
necessary, and
is taking the
time to get this
right," a Labor
Department
spokesperson
told CBS News on
Monday. This
official did not
offer any
timeline for
their decision.
"[The
president's]
objective is
actually to
protect workers
and members of
the workforce,"
White House
Press Secretary
Jen Psaki said
on Monday when
asked about the
OSHA
deliberations,
"We are waiting
for them to make
a conclusion."
Mr. Biden's
deadlines for
the Labor
Department on
this potential
face mask
standard landed
on his 55th day
in office, which
is half the time
he allotted for
other 100-day
goals, like
vaccinating
Americans and
returning
students to the
classroom.
As the emergency
standard remains
under review at
the Labor
Department,
dozens of public
health officials
and powerful
unions like the
AFL-CIO,
National Nurses
United and the
American
Federation of
Teachers
have pushed the
Centers for
Disease Control
and Prevention
to update their
scientific
guidance on the
aerosol spread
of COVID-19.
OSHA has the
Power to Set and
Enforce Mask
Standards
OSHA, a large
regulatory
agency within
the US
Department of
Labor, has
federal power to
set and enforce
standards to
ensure safe
working
conditions that
cover most
working
Americans in all
50 states. It
can enforce
requirements
anywhere from
local shops to
factory floors
to large
corporations.
Failure by a
business to
comply with any
OSHA requirement
can result in
fines, jail time
and legal
liability.
Federally
mandating masks
in all
workplaces is
unprecedented.
There were
previous
regulations on
respiratory
protection for
workers in
hazardous
environments or
healthcare
roles, but not
for the majority
of the American
workforce.
Earlier this
year, OSHA
issued guidance
encouraging
employers to
implement
measures to
prevent the
spread of
Covid-19 in the
workplace but
did not mandate
them to act.
That may soon
change.
A federal OSHA
workplace mask
regulation would
apply even in
states that are
no longer
requiring face
coverings or
have loosened
Covid-19
restrictions.
A US Department
of Labor
spokesperson
told CNN that
OSHA is
considering the
need for an
emergency
temporary
standard, or any
new rules and
regulations, to
better protect
workers during
the pandemic.
That includes
considering
rules for face
masks, and OSHA
is exploring
using the first
efficiency
standard for
consumer masks
that can
guarantee
quality and
effectiveness,
which were
recently
published by
ASTM
International --
an international
standards
organization.
"As OSHA studies
this potential
action, it will
consider the
recent ASTM
standard on
barrier face
coverings and
its potential to
provide the most
effective
personal
protective
benefits," a US
Department of
Labor
spokesperson
told CNN
Wednesday
Four Democratic
House committee
chairs
recently implored the
CDC and the
White House
COVID Response
team to do the
same, declaring
the government's
current science
on aerosol
spread was
"outmoded."
OSHA Could
Require Masks to
Meet the ASTM
Standard
ASTM
International
recently
published the
first national
mask standard
for consumers
that outlines
minimum fit,
design,
performance and
testing
requirements and
would require
user
instructions,
package labeling
and a permanent
tag on the
product. To meet
ASTM standards,
manufacturers
are required to
test their
facial coverings
in accredited
labs to certify
performance,
register their
products and use
an outlined ASTM
labeling system
on their
products.
Previously there
were no
standards for
consumer masks,
even though
masks are highly
recommended by
US health
officials to
help prevent the
spread of the
coronavirus.
While many
Americans have
been going to
work throughout
the pandemic,
some Americans
have yet to
return to their
workplace in a
Covid-19
environment. To
ensure workers
are safe, OSHA
is working to
expand the
ability to
report workplace
safety
complaints in
relation to
Covid-19
protection.
"We are
preparing to
implement a
national
emphasis program
that focuses our
efforts on
violations that
put the largest
number of
workers at risk
of contracting
coronavirus or
are contrary to
anti-retaliation
principles," a
Department of
Labor
spokesperson
told CNN
Wednesday. "OSHA
is reviewing its
enforcement
efforts related
to Covid-19 and
identifying
changes to
better protect
workers and
ensure the
safety of its
compliance
officers," the
spokesperson
continued.
Pressure has
been mounting on
OSHA to adopt
the new ASTM
mask standards.
In a letter to
Biden's top
coronavirus
advisers last
month, a dozen
health and
safety experts
-- including
four members of
Biden's former
advisory board
-- called on the
CDC and OSHA to
adopt the ASTM
standard for
better
protection for
workers and the
general public.
The letter asks
OSHA to create a
higher benchmark
for workers --
masks with 80%
protection -
using the design
and testing
criteria
outlined by the
ASTM standard.
Mask
Manufacturers
Cite Present
Production of
One Billion High
Efficiency Masks
per Month
Mask
manufacturers
are also
encouraging OSHA
to adopt the new
ASTM mask
standard. In two
recent letters
to the Biden
administration,
the Association
of the Nonwoven
Fabrics
Industry, known
as INDA, and the
newly formed
American Mask
Manufacturers
Association, or
AMMA, tell the
Biden
administration
that American
mask and
material
manufacturers
can adequately
supply the
country with
high-quality
masks and
encourage the
implementation
of a national
mask standard.
INDA represents
more than 200 US
suppliers of raw
materials and
equipment
manufacturers
and AMMA, is
made up of more
than 40 American
mask
manufacturing
companies.
"Based on INDA's
activities
working with US
government
entities and its
membership, it
is INDA's
opinion that the
raw material
shortage for the
production of US
face masks and
respirators has
been addressed
for the time
being," INDA
President Dave
Rousse said in
the letter,
which was sent
to President Joe
Biden.
The other
letter, by AMMA,
outlines the
current US
monthly
production
capacity of 902
million
high-filtration
masks and says
there are 690
million unused
high-filtration
masks currently
sitting in
warehouses.
"We want to
assure you that
America's mask
manufacturers
have ample
capacity to meet
the entire needs
of our nation
during
emergencies such
as this
pandemic," the
AMMA letter
says.
Italy Institutes
Lockdown with
Other EU
Countries Also
Pulling Back
A year after
Italy became the
first European
country to
impose a
national
lockdown to
contain the
spread of the
coronavirus, the
nation has
fallen eerily
quiet once
again, with new
restrictions
imposed on
Monday in an
effort to stop a
third wave of
infections that
is threatening
to wash over
Europe and
overwhelm its
halting mass
inoculation
program.
As he explained
the measures on
Friday, Prime
Minister Mario
Draghi warned
that Italy was
facing a “new
wave of
contagion,”
driven by more
infectious
variants of the
coronavirus.
Just as before,
Italy was not
alone.
“We have clear
signs: The third
wave in Germany
has already
begun,” Lothar
Wieler, head of
the Robert Koch
Institute for
Infectious
Diseases, said
during a news
conference on
Friday. Prime
Minister Viktor
Orban of Hungary
predicted that
this week would
be the most
difficult since
the start of the
pandemic in
terms of
allocating
hospital beds
and breathing
machines, as
well as
mobilizing
nurses and
doctors.
Hospitalizations
in France are at
their highest
levels since
November,
prompting the
authorities to
consider a third
national
lockdown.
Officials in the
United States
are watching
those
developments
with wary eyes.
At a White House
news briefing on
Monday, Dr.
Rochelle
Walensky,
director of the
Centers for
Disease Control
and Prevention,
pleaded with
Americans not to
let their guard
down as case
numbers have
dropped from
their peak. She
pointed to images
of young people
crowded onto
Florida beaches,
though generally
people are safer
outside than
inside, and to
European nations
as a warning.
“Each of these
countries has
had nadirs like
we are having
now, and each
took an upward
trend after they
disregarded no
mitigation
strategies,” she
said. “They
simply took
their eye off
the ball. I’m
pleading with
you for the sake
of our nation’s
health. These
should be
warning signs
for all of us.”
The U.S. death
rate remains
at nearly 1,400
people every
day. That number
still exceeds
the summer peak,
when patients
filled Sun Belt
hospitals and
outbreaks in states
that reopened
early drove
record numbers
of cases, though
daily deaths
nationwide
remained lower
than the first
surge last
spring. The
average number
of new reported
cases per day
remains
comparable to
the figures
reported in
mid-October.
Across Europe,
cases are
spiking. Supply
shortages and
vaccine
skepticism, as
well as
bureaucracy and
logistical
obstacles, have
slowed the pace
of inoculations.
Governments are
putting
exhausted
populations
under lockdown.
Street protests
are turning
violent. A year
after the virus
began spreading
in Europe,
things feel
unnervingly the
same.
In Rome, the
empty streets,
closed schools,
shuttered
restaurants and
canceled Easter
holidays came as
a relief to some
residents after
months of
climbing
infections,
choked hospitals
and deaths.
“It’s a
liberation to
return to
lockdown,
because for
months, after
everything that
happened, people
of every age
were going out
acting like
there was no
problem,” said
Annarita
Santini, 57, as
she rode her
bike in front of
the Trevi
Fountain, a
popular site
that had no
visitors except
for three police
officers. “At
least like
this,” she
added, “the air
can be cleared
and people will
be scared
again.”
For months,
Italy had relied
on a color-coded
system of
restrictions
that, unlike the
blanket lockdown
of last year,
sought to
surgically
smother emerging
outbreaks in
order to keep
much of the
country open and
running. It does
not seem to have
worked.
“History repeats
itself,” Massimo
Galli, one of
Italy’s top
virologists,
told the daily
Three EU
Countries
Suspend
AstraZeneca
Vaccine Use
Germany, France,
Italy and Spain
became the
latest countries
Monday to
suspend use of
AstraZeneca’s
COVID-19 vaccine
over reports of
dangerous blood
clots in some
recipients,
though the
company and
European
regulators have
said there is no
evidence the
shot is to
blame.
AstraZeneca’s is
just one of
three vaccines
in use on the
continent. But
the cascading
number of
nations raising
the alarm
amounts to
another setback
for the European
Union’s
vaccination
drive, which has
been plagued by
shortages and
other hurdles
and is lagging
well behind the
campaigns in
Britain and the
U.S.
The EU drug
regulatory
agency called a
meeting for
Thursday to
review experts’
findings on the
AstraZeneca
vaccine and
decide whether
action needs to
be taken.
The furor comes
as much of
Europe is
tightening
restrictions on
schools and
businesses amid
surging cases of
COVID-19.
Germany’s health
minister said
the decision to
suspend
AstraZeneca
shots was taken
on the advice of
the country’s
vaccine
regulator, the
Paul Ehrlich
Institute, which
called for
further
investigation
into seven cases
of clots in the
brains of people
who had been
vaccinated.
“Today’s
decision is a
purely
precautionary
measure,” Jens
Spahn said.
French President
Emmanuel Macron
said his country
will likewise
suspend shots
until at least
Tuesday
afternoon.
Italy’s drug
regulator
announced a
temporary ban,
less than 24
hours after
saying the
“alarm” over the
vaccine “wasn’t
justified.” And
Spain said it
will stop using
the vaccine for
two weeks while
experts review
its safety.
AstraZeneca said
there have been
37 reports of
blood clots out
of more than 17
million people
vaccinated in
the 27-country
European Union
and Britain. The
drug maker said
there is no
evidence the
vaccine carries
an increased
risk of clots.
In fact, it said
the incidence of
clots is much
lower than would
be expected to
occur naturally
in a general
population of
this size and is
similar to that
of other
licensed
COVID-19
vaccines.
The World Health
Organization and
the EU’s
European
Medicines Agency
have also said
that the data
does not suggest
the vaccine
caused the clots
and that people
should continue
to be immunized.
“Many thousands
of people
develop blood
clots annually
in the EU for
different
reasons,” the
European
Medicines Agency
said. The
incidence in
vaccinated
people “seems
not to be higher
than that seen
in the general
population.”
The agency said
that while the
investigation is
going on, “the
benefits of the
AstraZeneca
vaccine in
preventing
COVID-19, with
its associated
risk of
hospitalization
and death,
outweigh the
risks of side
effects.”
Blood clots can
travel through
the body and
cause heart
attacks, strokes
and deadly
blockages in the
lungs.
AstraZeneca
reported 15
cases of deep
vein thrombosis,
or a type of
clot that often
develops in the
legs, and 22
instances of
pulmonary
embolisms, or
clots in the
lungs.
The AstraZeneca
shot has become
a key tool in
European
countries’
efforts to boost
their sluggish
vaccine
rollouts. It is
also pillar of a
U.N.-backed
project known as
COVAX that aims
to get COVID-19
vaccines to
poorer
countries.
Pfizer’s and
Moderna’s
vaccines are
also used on the
European
continent, and
Johnson &
Johnson’s
one-shot vaccine
has been
authorized but
not yet
delivered.
In the U.S.,
which relies on
the Pfizer,
Moderna and J&J
vaccines,
AstraZeneca is
expected to
apply for
authorization in
the coming
weeks.
Denmark last
week became the
first country to
temporarily halt
use of the
AstraZeneca
vaccine. It said
one person
developed clots
and died 10 days
after receiving
at least one
dose. The other
countries
include Ireland,
Thailand, the
Netherlands,
Norway, Iceland,
Congo and
Bulgaria.
Britain and
Canada are
standing by
AstraZeneca’s
vaccine for now.
Dr. Michael
Head, a senior
research fellow
in global health
at the
University of
Southampton in
England, said
there is no data
yet to justify
suspending the
AstraZeneca
vaccine and
called the
decision
“baffling.”
“Halting a
vaccine rollout
during a
pandemic has
consequences,”
Head said. “This
results in
delays in
protecting
people, and the
potential for
increased
vaccine
hesitancy, as a
result of people
who have seen
the headlines
and
understandably
become
concerned.”
Spahn, the
German health
minister, said
of the decision
to suspend the
AstraZeneca
shot: “The most
important thing
for confidence
is
transparency.”
He said both
first and second
doses would be
affected by the
suspension.
German
authorities have
encouraged
anyone who feels
increasingly ill
more than four
days after
receiving the
shot — for
example, with
persistent
headaches or
dot-shaped
bruises — to
seek medical
attention.
Germany has
received
slightly over 3
million doses of
the AstraZeneca
vaccine, and
about half of
those have so
far been
administered,
compared with
almost 7 million
of the Pfizer
shot and about
285,000 from
Moderna.
The head of the
Spanish
Medicines
Agency, Maria
Jesús Lamas,
said Spain
detected its
first case of
clots last
Saturday. She
said the ban was
“not an easy
decision”
because it
further slows
the national
vaccination
campaign, but it
was the “most
prudent”
approach.
Almost 940,000
people in Spain
have received
the AstraZeneca
shot.
Europe,
meanwhile, is
reimposing
restrictions in
a bid to beat
back a
resurgence in
infections, many
of them from
variants of the
original virus.
In Italy, 80% of
children
nationwide
couldn’t attend
classes after
stricter rules
in more regions
took effect on
Monday. In
Poland,
bolstered
restrictions
were applied to
two more
regions,
including
Warsaw. Paris
could go into
lockdown in a
matter of days
because
intensive care
units are
getting swamped
with COVID-19
patients.
Halo Life Mask
Meets ASTM
Standard
The Halo Life
mask was also
recently
certified by
public safety
organization
ASTM
International.
This is a big
deal. The ASTM
released
much-needed standards for
face masks—which
are the first of
its kind—in
mid-February.
In order to be
certified by the
ASTM, companies
must show that
their masks meet
standards of
breathability
and fit and have
the ability to
filter out
particles at
least 0.3
microns, the
typical size of
aerosols that
contain viruses
and bacteria.
The Halo
Life face mask made
the cut and was
in the first
group of masks
to ever be
awarded this
certification.
The mask is
crafted with two
layers: A
polyester outer
layer with a
honeycomb look
and a lining
made of bamboo.
Why bamboo? It’s
cooling and
naturally
antibacterial,
so it can help
get rid of
germs and prevent
the mask from
getting smelly
with time and
repeat use. The
mask also
contains a
nanotechnology
filter that
captures more
than 99.8
percent of
airborne
particulates.
So, technically,
it’s a
three-layer
mask.
The mask itself
is durable, its
filter lasts for
more than 200
hours before it
should be
replaced. The
mask is usually
$35. Extra
filters are $4
each. So the
cost is 2 cents
per hour. If
masks were worn
1000 hours per
year the cost
would be $20 per
year.
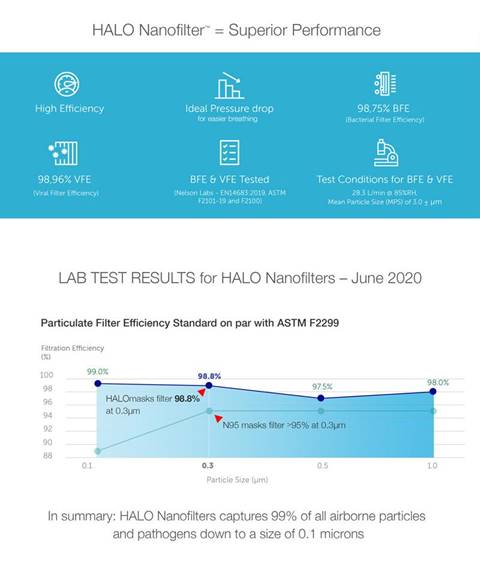
The performance
has been
verified by
Nelson
Laboratories.

