
Coronavirus
Technology Solutions
February 1, 2021
Step Two in the Three Step Mask Strategy Webinar
Friday Feb 5 at 10:AM CST
ISO is Also a Potential Resource in the Safe
Bubble Certification
SGS Galson Teams with SafeTraces to Supplement
PM with Aerosol Monitoring and Analysis
Certifying Relative Risk Reduction for a Safe
Bubble
Underwriters Laboratory Provides Mask Testing in
100 Countries
CDC Now Requires Masks on All Forms of Public
Transportation
Wyndham Grand Manana is SGS Certified
Clean & SAFE Protocol is a Certification Program
by SGS and HRS
Eurofins Tests and Certifies EU Community Masks
with Results Published on the Mask Package
Utah Governor Urges Upgrading Masks
__________________________________________________________________________
Step Two in the Three Step Mask Strategy Webinar
Friday Feb 5 at 10:AM CST
On the 28th we covered the background
for Step 1 of the Friday webinar. Today we are
covering Step 2 and tomorrow Step 3.
Click here to register for the February 5
webinar
https://home.mcilvainecompany.com/index.php?option=com_rsform&view=rsform&formId=92
The three steps are (l) launching an awareness
blitz, (2) advise on which masks should be worn
and (3) prioritize masks for the vulnerable
2. Communicate which type of masks people should
wear
a.
N95
b.
CATER
c.
surgical mask with brace
d.
eN95
e.
other
2.
Communicate which type of masks people should
wear
Cloth masks with a poor fit are now prevalent.
In general the poor fit allows 40% or more of
the virus to remain airborne. A number of
performance tests are shown in the Alerts. The
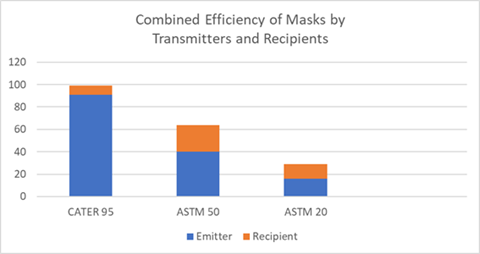
following chart shows the combined efficiency on
masks worn by both transmitter and recipient.
a.
N95
N 95 masks and their counter parts are efficient
and tight fitting. The questions revolve around
comfort and also availability.
b.
CATER
Comfortable, Attractive Tight Fitting,
Efficient, Reusable masks have all the factors
which are desired but there is a price for
comfort and attractiveness. However if these
masks are used for 40 days the cost is less than
$ 1/day.
The tight fit is critical. Some mask suppliers
such as Vogmask have five different sizes in
order to insure that the mask matches the face.
M^3D.ai has
a program discussed in our last webinar
and covered in the January 25 Alert where a mask
purchaser can use his mobile phone and select a
mask which will provide a good fit. They are
collaborating with Vogmask who has five
different mask sizes. Based on the phone image
the right size mask can be chosen.
Vogmask
has done extensive fit testing. If you
access the CMD intelligence system and search
under “fit” you find the following link
Quantitative inlet Fit by mask size.
This shows air in leakage under a variety
of motions for several mask sizes. This is the
quantity and quality of information that is
needed
by a consultant to properly rate a mask.
c.
surgical mask with brace
Surgical masks are readily available. The
meltblown media which is used is highly
efficient. The problem is the poor fit. As much
as 40% of the air can bypass the media. One
breakthrough is the Essential Brace which can be
used with the surgical mask to provide a tight
seal. This brace is designed for comfort as well
as sealing.
d.
eN95
The elastomeric N95 mask is a respirator with
replaceable filters and is now being used in
medical settings.
e.
other
There are other masks which could soon be
available.
If a tight fitting mask which
incorporates washable media can be made
available it could be an important option.
The three step program
requires availability of masks for
hundreds of millions of people quickly. Media
availability is one concern. Another is mask
making capability. With the mix of masks
available it is likely that the requisite masks
could be supplied during the second quarter of
this year.
Medical experts have recently stated that if
everyone had an N95 mask the pandemic would be
stopped in four weeks.
By the third quarter there could be a
combination of vaccinations and effective masks
which will provide the herd immunity in the U.S.
However it is unclear whether a vaccinated
unmasked individual can be a transmitter. If
this is the case then a combination of unmasked
vaccinated people and those with effective masks
is not an answer.
Once effective masking is achieved in Europe the
U.S. and developed countries there can be
greater focus on the underdeveloped
countries where herd immunity through
vaccination is not likely
for several years.
We invite you to join us on the 5th and discuss
these issues.
ISO is Also a Potential Resource in the Safe
Bubble Certification
ISO is a resource for consultants (experts) to
consider in the whole safe bubble program. ISO
has information on mask evaluation based on the
oxygen consumption of the individual.
The resistance tests now used are based on a
standard amount of air and constant flow. A mask
with a given filtration area can be used by
individuals whose quantity of oxygen consumption
and inspiratory flow varies considerably.
So the actual resistance will be
different depending on the individual.
ISO (the International Organization for
Standardization) is a worldwide federation of
national standards bodies (ISO member bodies).
The work of preparing International Standards is
normally carried out through ISO technical
committees. Each member body interested in a
subject for which a technical committee has been
established has the right to be represented on
that committee. International organizations,
governmental and non-governmental, in liaison
with ISO, also take part in the work.
One part
of ISO/TS 16976 provides
information on factors related to human
anthropometry, physiology, ergonomics, and
performance for the preparation of standards for
performance requirements, testing, and use of
respiratory protective devices. This part of
ISO/TS 16976 contains
information related to respiratory and metabolic
responses to rest and work at various
intensities. Information is provided for the
following:
·
— metabolic rates associated with various
intensities of work
·
— oxygen consumption as a function of metabolic
rate and minute ventilation for persons
representing three body sizes
·
— peak inspiratory flow rates during conditions
of speech and no speech for persons representing
three body sizes as a function of metabolic
rates.
The information contained within this part of ISO/TS 16976 represents
data for healthy adult men and women of
approximately 30 years of age but is applicable
for the age range of the general population.
SGS Galson Teams with SafeTraces to Supplement
PM with Aerosol Monitoring and Analysis
Just a few years ago, SGS Galson, under Ron
McMahan’s leadership, introduced SmartSense continuous
monitoring with sample capture, SGS Galson’s
state-of-the-art continuous monitoring system.
SmartSense is being used in many applications,
including PM monitors for wildfire particulate
monitoring. Now, SGS is combining a powerful
combination of air quality testing and
SmartSense continuous monitoring for IAQ to
reduce possible SARS-CoV-2 spread through
monitoring ventilation effectiveness.
SGS Galson is teaming with SafeTraces, a leader
and innovator in DNA-enabled diagnostic
solutions, to offer veriDART, the first
diagnostic solution for verifying ventilation
and filtration-focused engineering controls,
specifically for infection control, through a
novel methodology that safely mimics aerosol
mobility and exposure levels with DNA-tagged
tracer particles. More details on veriDART are here.
When scientists confirmed that aerosol spread of
the SARS-CoV-2 virus in indoor air presents a
significant risk to occupants, SGS Galson
provided an RT-qPCR method to monitor the virus
in air. One unresolved issue in the built
environment, however, was verifying whether
engineering and HVAC controls were effectively
reducing airborne virus indoors.
THE SOLUTION
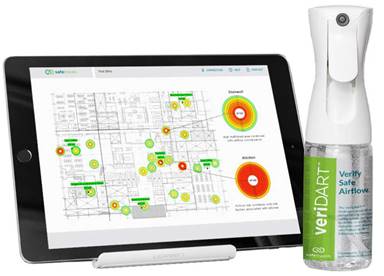
Teaming with SafeTraces, a leader and innovator
in DNA-enabled diagnostic solutions, SGS Galson,
now has available a patent pending kit-sampling
solution developed by SafeTraces,
the veriDART,
which verifies HVAC and filter systems for
aerosol transmission risk. SGS Galson’s
reputation for analytical excellence and the FreePumpLoan™ program
create a powerful combination with veriDART at
identifying SARS-CoV-2 transmission in the built
environment.
Facilities obtain
fast, accurate and cost-effective
analysis to reduce airborne viral accumulation
indoors.
The veriDART leverages customized test plans
that address customers’ assessment needs,
including survey tests, dilution ventilation
tests, and filtration tests.
The kit includes SafeTraces’ innovative aerosol
spray. This liquid aerosol-based solution uses
DNA tracers to safely mimic the composition and
mobility of viral aerosol to measure the
effectiveness of ventilation and filtration for
aerosol contaminants.
FOUR-STEP PROCESS
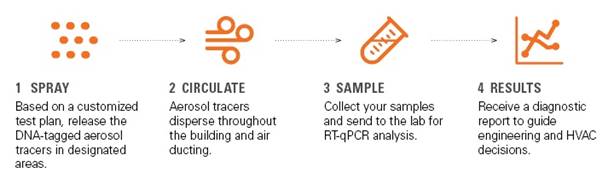
Since 2015, SafeTraces has been a leader and
innovator at the forefront of DNA-based testing
solutions for safety, traceability, and
environmental monitoring. Spawned from the
Lawrence Livermore National Labs, our
groundbreaking, patented technology leverages
DNAtagged particles that safely mimic the
mobility of airborne pathogens. In its early
days, our technology supported biosecurity-focused
applications for the Department of Homeland
Security and New York City Transit Authority, as
well as well as the Department of Defense.
Subsequently, the National Institutes of Health
(NIH) has supported SafeTraces with multiple
research grants to build on our initial
breakthroughs for wider development and
application of our technology in food,
pharmaceuticals, and the built environment.
Additional financial support has been provided
by the U.S. Food and Drug Administration (FDA)
and National Science Foundation (NSF), and
technical advisory support has been provided by
a world-class team of multi-disciplinary experts
in biochemistry, fluid dynamics, virology, and
building science at Stanford University, the
Massachusetts Institute of Technology (MIT), and
the University of Nebraska.
Chemical Composition: veriDART’s tracers mimic
the chemical composition of human saliva and
aerosols. Tracers consist of distilled water,
food-grade, water soluble ingredients, and DNA.
veriDART adheres to the highest levels of
product safety. The FDA confirmed Generally
Recognized as Safe (GRAS) status for the
technology
uses short, non-coding, non-living DNA
sequences. veriDART complies with OSHA, NIOSH,
and ECHA safe exposure limits.
Aerosol Mobility: veriDART’s tracer mobility
simulates transmission of airborne pathogens via
a spraying action that approximates human
coughing and sneezing and an air sampling action
that approximates human inhalation. Each spray
creates a distribution of tracer particle sizes
consistent within human respiratory droplet and
aerosol range. Meanwhile, veriDART employs an
air sampler with a vacuum flow rate similar to
breathing that pulls airborne particles on to a
filter specialized for small aerosols.
veriDART’s secondary sampling method is surface
swabs for customers interested in analyzing
fomite transmission risk.
Detection Levels: veriDART’s detection levels
are informed by infectious viral loads for
respiratory droplets and aerosols, with DNA
concentrated in tracers based on the latest
virology for SARS-CoV-2 and other airborne
pathogens. Leveraging polymerase chain reaction
(PCR) technology, veriDART measures the
difference between the baseline concentration
level of DNA copies in each tracer solution and
the detection level of each tracer solution
found at each sampling point in order to
establish a quantifiable reduction on a log10
scale. This log reduction is the basis for the
risk thresholds used in veriDART’s heat map
visualizations. A 3-log reduction, or 1000-fold
decrease, in DNA copies from the baseline to the
sampling point is considered the diagnostic
indicator for low risk.
Certifying Relative Risk Reduction for a Safe
Bubble
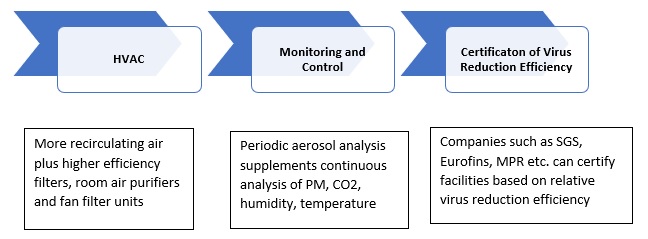
A number of companies rely on their expertise and knowledge to certify the performance of products. McIlvaine has been establishing a basis where facilities could receive a certification that their virus reduction initiatives meet the equivalent of any regulation.
The case of a facility where cloth masks are
allowed and
occupancy is limited to 25% was used as an
example. A certification can be provided that a
specific facility with 100% occupancy but tight
fitting masks and superior HVAC creates a risk
lower than the regulatory specified
risk.
The companies providing this certification can
just be consulting companies. Alternatively they
can be companies which also
provide some of the products and
services. In the above article we covered the
SGS collaboration to provide HVAC aerosol
analysis. This means that SGS is
already
involved in each of the nine steps which are
relevant in creating a certification basis
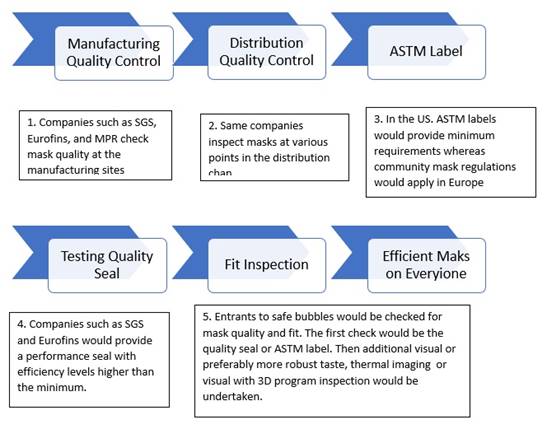
One advantage that a company such as SGS has is
that it can move quickly to provide
certification.
Most consulting companies would need some
time to organize the collaboration
and expertise necessary to do this. SGS
and Eurofins are also global companies which is
an advantage.
On the other hand these companies may be
too big and slow moving to meet the urgent
needs.
This leaves the door open for dynamic
consulting companies.
Underwriters Laboratory Provides Mask Testing in
100 Countries
Understanding and meeting the regulations
relevant to PPE products in all of the target
markets can be especially difficult during a
time of crisis. It requires both technical
expertise and industry know-how to navigate
regulatory complexities and bring
PPE products to market quickly.
Underwriters Laboratories evaluates PPE and
apparel to the industry’s standards for safety
and performance, including regulations and
guidelines provided by the World Health
Organization COVID-19 Operational Support and
Logistics Disease Commodity Packages, the U.S.
Centers for Disease Control and Prevention
(CDC), the U.S. Food and Drug Administration
(FDA) and the European Commission. UL test and
certify a wide range of PPE products including:
-
Protective goggles and face
shields, aka eye protection,
which are intended to mainly
protect the user’s eye or facial
area from emitted droplets to
ANSI Z87.1 in North America and
to EN 166 for Europe
-
Isolation gowns, surgical gowns
and drape protection for
healthcare providers to
ANSI/AAMI PB70 for North America
-
Partnering with external labs to
test medical gloves, which are
intended to protect the wearer
from contaminants during medical
examination and diagnostic
procedures, to ASTM D6319 in
North America
-
Partnering with external labs to
test filtering facepiece
respirators, also known as
respirators, N95 respirators or
particle-filtering half masks (FFRs)
to 42 CFR Part 84 (N95) and 21
CFR 878.4040 in North America
and to EN 149 for Europe
-
Surgical masks, nonsurgical
masks and nonmedical masks
UL
global solutions for both North America (UL
Mark) and Europe (CE Mark) help clients
demonstrate
compliance with PPE standards. UL will help
understand the guidelines and regulations
applicable to PPE products, test products to
determine compliance, and offer document review
for COVID-19 products certified in another
country. For new product types in consideration,
UL can support clients with the necessary
regulatory, technical and testing advice –
including 3D printing considerations – that can
help
bring innovative products to market
quickly.
Global guidelines for face mask testing may
leave retailers, brands and manufacturers
confused or unaware of the latest requirements.
UL face mask testing services can help take the
complexity out of the
supply
chain. UL says that as a trusted partner, it can
help expedite production of these essential
products to serve public health needs.
It provides solutions for three distinctive
types of face masks:
-
Surgical masks – medical masks
with liquid barrier protection
-
Nonsurgical masks – medical
masks with no liquid barrier
protection
-
Nonmedical masks – masks for
general public use
Solutions range from product and packaging
testing to assessments on labeling and
flammability. It can also provide factory audits
and inspections.
Benefits of face mask testing
When conducting product testing and
verification, UL
consider many regulations and standard
entities, including the U.S. Food and Drug
Administration (FDA). UL also offers quality
assurance programs that evaluate an entire
operation and can provide facility inspections
and training. Consumer and health care workers’
safety and well-being are paramount to the
program, and
mission to support public safety and
health.
Third-party inspections of consumer goods are a
key checkpoint in a global quality assurance
program. They help ensure the product meets
specification before release and shipment and
help build and maintain consumer trust.
Why UL?
-
UL operates in more than 100
countries and its reports and
certifications are recognized
and accepted around the world.
-
UL has
helped to set more than
1,600 Standards defining safety,
security, quality and
sustainability.
-
UL knowledge of global standards
and regulatory requirements
covers many markets and
authorities including the U.S.
Food and Drug Administration and
Centers for Disease Control and
Prevention.
CDC Now Requires Masks on All Forms of Public
Transportation
Starting this week, travelers and commuters will
be required to wear face masks on nearly all
forms of public transportation as part of a
sweeping new order from the Centers for Disease
Control and Prevention aimed at slowing the
spread of the coronavirus.
The order, issued late Friday, will require
masks to be worn by "all passengers on public
conveyances" traveling into or within the United
States, including airplanes, ships, ferries,
trains, subways, buses, taxis and ride-shares.
Coverings will also be required at
transportation hubs like airports, bus
terminals, and train or subway stations. The new
guidelines take effect at 11:59 p.m. ET on Feb.
1.
"Requiring masks on our transportation systems
will protect Americans and provide confidence
that we can once again travel safely even during
this pandemic," said the
11-page order signed
by Dr. Martin Cetron, director of the CDC's
Division of Global Migration and Quarantine.
"Therefore, requiring masks will help us control
this pandemic and aid in reopening America's
economy."
The new order arrives at a pivotal moment in the
pandemic. One year to the day since the World
Health Organization declared the coronavirus a
global health emergency, the U.S. has seen nearly
26 million cases and
more than 436,000 deaths. And while two vaccines
have already begun rolling out, troubling
new variants from
South Africa and the United Kingdom are raising
growing alarm about whether health officials can
prevent the virus from spiraling even further
out of control.
Friday's order helps underscore the importance
the new administration is placing on masks as
part of that effort. Under President Donald
Trump, the CDC was blocked from
requiring masks on public transportation, but on
his first full day in office President Biden signed
an executive order requiring
passengers to wear face coverings during
interstate travel.
"The experts say by wearing a mask from now
until April, we'd save more than 50,000 lives
going forward," Biden said.
The CDC order adds several layers of new detail
on top of Biden's executive action, listing as
one of its four main objectives "the
preservation of human life."
The guidelines allow for a handful of
exceptions. Children under the age of two won't
be required to wear coverings, and neither will
anyone with a disability who cannot safely wear
a mask. Otherwise, the order says passengers and
operators are required to wear their masks at
all times except "for brief periods," such as to
eat, drink or take medications.
The agency said that airlines and other
operators must "at the earliest opportunity"
remove any passenger who refuses to comply with
the mask order. Anyone violating the order could
face potential criminal penalties, but the CDC
suggested that civil penalties would be more
likely
Wyndham Grand Manana is SGS Certified
The hotel has the following
statement on its website “SGS is the
world’s leading inspection, verification,
testing and certification company. They are
recognized as the global benchmark for quality
and integrity. With more than 89,000 employees,
SGS operates a network of more than 2,600
offices and laboratories around the world.
The “Disinfection Monitored, Cleaning Checked
Mark” certification is includes an on-site
inspection couple with fluorescent marking and
ATP sampling and testing. The methodology
validates, amongst others, the effectiveness of
cleaning procedures, the adequacy of products
used and the implementation of enhanced cleaning
schedules, especially on high frequency touch
points.
For your safety and protection, the hotel, its
rooms, facilities and common areas are regularly
cleaned, disinfected and sanitized according to
specifically designed guidelines. Count on us to
put your safety first!”
SGS is described by the hotel as a global
benchmark of quality and integrity. Many of the
disinfection analyses resulting in
certification are qualitative. They are more
qualitative than
comparing a facility virus reduction
percentage compared to a regulatory standard
such as 25% occupancy.
Clean & SAFE Protocol is a Certification Program
by SGS and HRS
HRS, the leading global corporate lodging
platform, and SGS have a
cleanliness-focused
program for the hotel industry.
The Clean & Safe
Protocol provides corporations and hoteliers
with a well-defined standard at a time when
property hygiene is the leading factor as
corporations plan to send business travelers
back on the road. Hotels earning the designation
as a safe property gain access to a new label
for use in HRS procurement and booking channels,
their own corporate website, and on-property
displays at entrances, lobbies and in-room
marketing. HRS will display this label on its
proprietary desktop, mobile and agent booking
solutions, as well as corporate online booking
engines (OBEs) such as Concur and Cytric.
HRS is the leading global provider of hotel
procurement services, managing hotel
negotiations for more than one-third of the
Fortune 500. In
a
survey of its corporate customers,
86 percent of
companies reported that they will only engage
with hotels that have implemented revised
specific COVID-19 hygiene measures.
In the wake of the COVID-19 pandemic and the
dramatic pause in business travel, cleaning and
safety standards for travelers have become the
most important deciding factor for corporations
using the downturn to recalibrate hotel programs
for the balance of 2020 and 2021. As countries
loosen travel restrictions and domestic business
travel gains momentum, a
hotel's ability to showcase its enhanced hygiene
protocol is crucial for
gaining more business guests and better
long-term relationships with preferred corporate
clients. Hotels can use the Clean & Safe
Protocol not only to convey their enhanced
hygiene practices, but also to make booking
easier for business travelers, as the labels
enable them to compare inspected hotels before
making a selection.
The Clean & Safe Protocol is based on a
comprehensive catalogue of measures that
includes recommendations from the World Health
Organization (WHO), the World Travel and Tourism
Council (WTTC) and the Centers for Disease
Control and Prevention (CDC), as well as
guidelines for meetings and groups as defined by
the Research Institute for Exhibition and
Live-Communication (RIFEL). Measures include:
-
Improved hygiene services in
public areas, guest and meeting
rooms
-
Extended infection prevention
measures
-
Guaranteed minimum distances
-
Implementation of revised
training programs for employees
and suppliers
-
Regular control and monitoring,
and
-
Other measures that
illustrate consistent, recurring
practices that enhance safety
Many hotels worldwide have already enhanced
their hygiene measures to address the new
reality, with chains leading the way. HRS and
SGS are committed to helping all hotels –
multi-national chains, regional groups, and
independent properties – ensure their
cleanliness and distancing investments pay off
via accurate representation in procurement and
shopping channels. By conveying each hotel's
enhanced practices, business travelers and hotel
program leaders will quickly gain confidence as
they look
ahead to re-launching travel activities.
By incorporating
internal processes and employee security, the
Clean & Safe Protocol also demonstrates the
hotel's focus on both employees and guests.
The Clean & Safe Standard gives all hotels the
opportunity to demonstrate their safe hygiene
practices. As a first step, hotels can engage
with HRS to fill out an online self-assessment
form. Hotel groups can bundle forms for their
affiliated properties. If the hotel fulfills the
necessary requirements, it receives a "Clean &
Safe Self Inspected" label on all HRS
procurement and booking channels. These hotels
can also use these channels to show the multiple
steps they are taking to enhance cleanliness and
on-property distancing.
Secondly, HRS and SGS offer hotels the
opportunity to have new measures inspected and
confirmed via remote digital inspection or
onsite by an SGS inspector. Upon passing the
inspection, the hotel receives the "Clean & Safe
Expert Inspected" label, which is most relevant
for corporate procurement leaders and travelers.
This can be displayed on the hotel property and
website. Hotels that have implemented their own
measures and had them validated by an external
audit partner will also receive the "Clean &
Safe Expert Inspected" label directly with
appropriate proof.
"COVID-19 has fundamentally changed the world of
business travel. In times of pandemic and
beyond, corporate travel managers are rigorously
weighing their duty of care responsibilities and
traveler safety as their colleagues get back on
the road to see clients and sell to prospects,"
says Tobias
Ragge, CEO of HRS.
"Hoteliers around the world are investing
significant amounts into new cleanliness
procedures. We intend to make their efforts
transparent, as this is needed to restore the
sentiment of trust for business travelers and
corporate buyers. SGS, a recognized provider in
the field of corporate lodging with its leading
inspection and auditing offerings and a truly
global footprint, is an ideal partner on this
important issue for our corporate customers,
hotel partners and business travelers."
"SGS's global leadership in the travel and
hospitality industry has enabled our network of
health and safety experts to develop a
comprehensive and straightforward protocol in
100+ countries for reviewing hotel management
procedures and disinfection status," said Frankie
Ng, CEO of SGS. "With
HRS, we have a great strategic partner to
advance
Eurofins Tests and Certifies EU Community Masks
with Results Published on the Mask Package
As the use of community masks –also referred to as hygienic masks - becomes part of our daily lives, the European Committee for Standardization (CEN) has published, by request of the European Union, the Workshop Agreement (CWA) guidelines CWA 17553.
Eurofins offers a service of testing against the
minimum requirements. This includes
• Visual inspection - Tears, harness detachment,
ill-fit, deformation, etc.
• Size - Adapted to the morphology of the
average user.
• Materials – should withstand handling and wear
throughout the claimed lifetime.
• Cleaning – if reusable, shall withstand at
least 5 cleaning cycles according to EN ISO
6330.
• Surface condition of the parts - No sharp
edges or burrs.
• Filtration efficiency of the material –
Checked against the two determined levels (90%
and 70%), based on the efficacy to filtrate
particles (3 ± 0.5 μm).
• Head harness – Self-adjusting, elastic or with
laces, over the head or ears and designed to fit
the average user, the harness should be robust
enough while avoiding discomfort. It shall
withstand 5 donning and doffing cycles by at
least three subjects with different
morphologies.
• Breathing resistance and air permeability –
The material shall not exceed a differential
pressure ≤ 70 Pa/cm² (correlates to 80 l/s/m²)
for a vacuum pressure of 100 Pa.
Marking
checks. Marking
on the product packaging is mandatory, in the
concerned official domestic language(s).
Eurofins’ label and marking check service
includes:
• Completion of name, trademark, address and
contact details of the responsible actor
(producer or importer).
• Product batch ID.
• If tested against the CWA document,
designation “Community face coverings CWA”
followed by the version of the concerned CWA
document.
• The
filtration efficiency level, method used and
standard. • Durability - “reusable” or
“disposable”. • Targeted user age, “child” or
“adult”.
• Restriction warning: “Not suitable for
children under 3 years old
Utah Governor Urges Upgrading Masks
With the more contagious U.K. variant of
COVID-19 circulating in Utah and widespread
vaccination months away, Gov. Spencer Cox says
he would “encourage people, if you can, to
upgrade the quality of your masks.”
Utah is working with the Biden administration,
he added at a Thursday news conference, “on
getting more and better masks out to people ...
The right quality mask protects the user as well
as those around them.”
So should Utahns double up like inaugural poet
Amanda Gorman, who wore a
plain mask under her sparkly red rhinestone one?
Or set aside their
cloth facial fashions for medical-quality gear?
Double masking often means wearing a surgical-stye
mask under a cloth mask. And it does have
potential benefits for those who want to do all
they can to protect themselves, or anyone in a
higher risk environment like public
transportation, said Dr. Russell Vinik, chief
medical operations officer at University of Utah
Health.
Public health expert Dr. Anthony Fauci has been explaining
in interviews about double masking that
“the more material, the more filters, the more
virus that’s going to get trapped in those
filters,” Vinik said.
“In addition, double masking does create a
better fit,” Vinik said in an online briefing
hosted by the U.
“On the other hand, what we know is, masking
works,” Vinik emphasized. “And more than
anything, our message to the public is, wear a
mask and wear a mask properly. Wear it over your
nose ... wear it all the way under your chin.”
With the arrival of COVID-19 variants, the
conversation around masking is shifting to, “OK,
how do we make masks more effective?” said Dr.
Eddie Stenehjem, an infectious disease
specialist at Intermountain Healthcare.
“Up until now, it’s just been ‘wear a mask.’
There hasn’t been any kind of discussion about,
is it a quality mask? Is it a mask that actually
fits on your face?” Stenehjem said. “Is it one
that has a nose bridge, and actually stays
there? Or does it move when you talk?”
People need to ensure their masks fit well and
offer multiple layers of material to create an
effective barrier, he said. “If that means
wearing a double mask, or a surgical mask with
something over it, I think that’s a great way to
go.”
During a community update offered by
Intermountain,
Stenehjem also broke down the types of masks
available and their effectiveness.
N95 masks
• These
are costly, in short supply, and needed by
health care workers who are caring for people
with coronavirus, Stenehjem said. “An N95 mask
is a very tight-fitting mask; you have to have
it fit to your face to ensure that it has a
functional seal,” he said. They are the best
masks for preventing the transmission of
respiratory droplets that carry the virus, he
said.
Right now, with all personal protective
equipment for Utah caregivers — N95 masks,
gowns, gloves — “we’re in a pretty decent spot,”
he added. Health care providers have to be
prepared for any possible future surges, he
noted, so they are careful to maintain their
supplies.
KN95 masks
• These
masks, which are becoming more available, aren’t
fitted to an individual’s face, Stenehjem said.
“They are still a very good mask, though, in
terms of preventing respiratory transmission.”
Hospital-grade surgical masks
• This
is the type of mask Stenehjem wears daily to
talk with hospitalized patients and even care
for coronavirus patients who are not on a
ventilator or undergoing respiratory procedures,
he said. They are meant for health care workers
and can be worn all day, he said.
Consumer surgical masks
• Standard
“one and done” surgical masks that can be found
at Costco and other outlets are the next step
down, he said.
Pandemic-era masks
• “And
then you have all these other masks that have
been created, right, whether it be through
Cotopaxi or through bandanas or through buffs,”
he said. Their quality can be unknown, yet
“that’s what the majority of people wear,” he
said.
Besides double masking, Utahns thinking about
improving their mask quality could consider the
disposable, daily surgical masks, he said, or
any masks with multiple layers, that have a good
seal over your nose, that cover your face and
fit well.
For now, the Utah Department of Health has not
changed its guidelines for mask users, a
spokesman said.
“We recommend people wear two or more layer
cloth masks that cover the nose and mouth,” he
said in an email. “There is no recommendation
that people wear surgical, N95, or KN95,
although if people want to do so that is
certainly their prerogative.
