
Coronavirus
Technology Solutions
January 29, 2021
Three Step Mask Strategy Webinar Friday Feb 5 at
10:AM CST
RDI Medical has High Speed Mask Making Machinery
China Reports COVID Outbreak At Chicken
Processing Plant
_______________________________________________________________________________
Three Step Mask Strategy Webinar Friday Feb 5 at
10:AM CST
On Friday, February 5 we will discuss the three
step plan authored by
-
Devabhaktuni Srikrishna is
the founder of Patient
Knowhow,
-
Joseph Buccina is
a director at In-Q-Tel’s B.Next,
-
Dan Hanfling,
MD, is a clinical professor of
emergency medicine at George
Washington University, co-chair
of the National Academy of
Medicine Forum on Medical and
Public Health Preparedness, .
-
Monica Gandhi,
MD, is an infectious disease
specialist, professor of
medicine, medical director of
the Ward 86 HIV Clinic, and
director of the Center for AIDS
Research at the University of
California, San Francisco.
-
Donald Milton,
MD, is a professor of
environmental and occupational
health at the University of
Maryland School of Public
Health. \xxx.
Sri will provide a brief overview of the plan
but we also ask attendees to view details in the
January 27 Alert. We will discuss each of the
three steps and contents below. We encourage
you to participate and also to submit evidence,
opinions etc. ahead of time.
As Sri and the other authors point out the three
step plan needs to be quickly implemented and
“it is up to CEOs, mayors, and governors to
implement these three steps to ensure consistent
use of the best possible masks for vulnerable
groups”. Here is a proposed agenda of subjects
to be addressed.
The three steps are (l) launching an awareness
blitz, (2) advise on which masks should be worn
and (3) prioritize masks for the vulnerable.
In the Alert today we will be providing
background data for the webinar on the first
step. Background data for the other steps will
be in the Alerts next week
The three steps are (l) launching an awareness
blitz, (2) advise on which masks should be worn
and (3) prioritize masks for the vulnerable.
1.
Launch an awareness blitz
a.
roles
i.
mainstream media including local news outlets
ii.
role of association and occupational media
including business
and medical
iii.
suppliers from the large validators such as SGS
and Eurofin to media suppliers to mask
manufacturers
iv.
CATER Mask Decisions
b.
message
i.
push - the need to mandate use of efficient
masks
ii.
pull - the advantage of using pull or incentives
1.
the safe bubble with the promise of full
occupancy within
the bubble
iii.
criteria
1.
general and absolute
2.
relative risk reduction as proposed by Mcilvaine
iv.
product availability
1.
media
2.
masks
3.
timing
4.
geographies
a Roles -BARDA
BARDA DRIVe, in partnership with the National
Institute for Occupational Safety and Health
(NIOSH), seeks to understand the barriers and
challenges associated with the next generation
of innovative mask designs. The Mask Innovation
Challenge is an initiative that aims to support
the development of innovative masks that can
protect Americans from respiratory pathogens.
McIlvaine submitted a proposal as requested by
BARDA.
Here are excerpts
Response to BARDA RFQ by the McIlvaine Company
Thanks for the opportunity to submit a proposal
relative to public masks. McIlvaine has
published Decision Systems for nearly 45
years. These have all been services paid for by
the users.
Due to the magnitude of the problem and
the realization that tight fitting efficient
masks would be the solution McIlvaine initiated
a free Decision System
on mask options to mitigate COVID in
March 2020. Daily Alerts webinars and an
intelligence system make this service an ideal
vehicle to accomplish the ends indicated in the
RFI.
Access to the system is available at
http://www.mcilvainecompany.com/CATER/subscriber/default.htm
What do you see as the most important criteria
to evaluate the effectiveness of a community
mask that is to be used by the general public?
Examples might include assurance of consistent
fit, comfort, airflow resistance, ability to
protect the wearer and stop particles from
escaping the mask, cost, etc. .
Answer: We are addressing these questions on a
daily basis in our Coronavirus Mask Decisions.
One segment has been grouped as CATER
which stands for Comfortable Attractive, Tight
fitting, Efficient, and Reusable. Another group
has been identified as disposable efficient
and tight fitting. Another group is
disposable, efficient, and loose fitting. A
fourth group is disposable, efficient, loose
fitting but with a brace which makes them tight
fitting. A fifth group is industrial
respirators.
The most important criterion is to provide
everyone with tight fitting efficient masks.
This can be done within just a few months with a
combination of the above mask types and initial
heavy reliance on medical masks made tight with
a brace. The goal is to fit everyone with a mask
which has a FFE (fitted filter efficiency) of
90%.
Extensive quantitative testing has shown that a
surgical mask with a brace can equal an N95
mask. An N95 mask can achieve a 98% fitted
filter efficiency. Billions of surgical masks
are being produced. If the wearers also used the
reusable brace they
could average 90% FFE. So 10% emission
from the transmitter and 90% removal by the
recipient means that he inhales only 1% of the
virus. This 99% virus reduction is more
protection than vaccines and would allow quick
return to normal activity
What
sectors or stakeholders should BARDA engage as
part of these efforts?
Answer:
Engagement should extend to associations,
governments, fiber suppliers, media
manufacturers, mask manufacturers, antimicrobial
suppliers, quality control consultants,
suppliers of particulate measuring
instrumentation, fit testers, and end user
groups from fitness centers to schools. Meat
processors and other industrial end users should
also be involved.
McIlvaine has identified decision makers
at many of these entities and is conveying their
insights in the Alerts and in periodic webinars.
If there is an opportunity for sponsorship, is
your organization interested in participating as
a potential sponsor? If yes, what are the
capabilities of your organization in support as
a potential sponsor?
Coronavirus
Mask Decisions covers all masks for
public use.
It is presently a free service with Daily
Alerts, webinars and an intelligence system with
links to relevant papers. It can be accessed at
http://www.mcilvainecompany.com/CATER/subscriber/default.htm
McIlvaine is willing to partner with BARDA in a
range of ways. At a minimum it could include all
data as directed by BARDA in the system.
At a maximum Coronavirus Mask
Decisions could be become a government owned
initiative with McIlvaine as
sub-contractor as directed.
Would your organization be interested in joining
a list of experts that may provide mentorship to
challenge winners?
Answer: Yes
Would your organization be interested in serving
as a marketing partner and provide marketing and
outreach as part of the challenge competition
including posting on websites, social media, and
other outlets.
Answer: Yes. McIlvaine has capabilities well
beyond the those involving Coronavirus Mask
Decisions. It has databases and reaches
100,000 people around the world who have air,
water, and energy interests. A number of
contracts have been executed with U.S. and other
governments relative to environmental issues.
Bob McIlvaine was hired by EPA to testify before
senate sub committees relative to SO2 emissions
and solutions. He was also a director for 25
years of the Institute of Clean Air Companies.
McIlvaine works closely with associations. The
feature article by McIlvaine in the upcoming
issue of International Filtration News is
on masks. This publication is owned by INDA
(association of non-woven manufacturers).
McIlvaine writes feature articles each month for
more than seven publications.
In addition it has more than 50 of its
own publications.
i Main stream media including local news outlets
White papers:
Sri has been quoted in a number of main stream
publications relative to the benefits of
efficient masks. However, some of these articles
include contradictory comments or ones that are
misleading.
A series of whitepapers could be made
available to the media along with contacts who
could be interviewed relative to the contents.
For example one big question is the availability
of enough media. A white paper coming from INDA
on this subject with contacts for journalists
would be very helpful.
Coordination with important journalists:
Sri has important contacts as do others in the
webinar.
McIlvaine has a long relationship
supplying environmental assessments to Keith
Bradsher, Shanghai Bureau Chief of the NY Times.
This resulted in a long article on Chinese
meltblown capacity in the NY Times a few
months ago. But McIlvaine has not had success
with the U.S. journalists.
ii Role of association and occupational media
including business and medical
The white papers on specific subjects will be of
interest to the business and medical media. The
role of international associations such as EDANA
are also important. Masks need to be viewed as
one of the air cleaning tools and should be
considered by the Institute of Clean Air
Companies, American Filtration Society, Waterloo
Filtration Institute and others.
iii Suppliers from the large validators such as
SGS and Eurofin to media suppliers to mask
manufacturers
Coordination among suppliers to provide
a consistent and powerful message will be
beneficial. Companies
can be promoting their own products as part of
the solution. The awareness blitz should be
aimed at specific groups such as meat
processors, schools, hotels etc.
McIlvaine has email contacts at certain
of these groups. A webinar for meat processors
featuring presentations by a number of suppliers
will be advantageous
iv CATER Mask Decisions
As proposed to BARDA above Cater Mask
Decisions can be a free analytical tool.
There is an intelligence system with summaries
of articles as well as the daily alerts and
webinars. There are two search engines.
b. Message
i
Push - the need to mandate use of efficient
masks
Erick Couch is charting a course for public
acceptance of more efficient masks using the
ASTM label as a major selection criterion.
Eric believes a power point presentation which
is viewed at http://home.mcilvainecompany.com/images/Roadmap_to_High_Performance_Masks-BCTF.pdf
provides a meaningful depiction that can be
comprehended by the general public...illustrated
ranges with indicators for performance of N95s,
cloth face.
ASTM –
Respected Industrial Standard Body
Essence:
Allows use of widely available materials that
provide high performance filtration without
impacting N95 supply to frontline responders.
New and Existing Mask Fabricators Can Scale
Supply within 6 Weeks
Performance Measures
Sub-micron Particle Filtration (i.e. 95%)
Breathability
Inward Leakage Assessment
Eliminates Lengthy Testing / Approval Process
Chaired by CDC Deputy Director Jonathan Szalajda
Impact: Immediately contrasts surgical and
cottage industry non-sealing masks with a high
performing masks made to measurable standard.
• Jan 4 Vote Jan 12 Special
Meeting Feb 12 Release
NOTE: Establishes measurement method, defines
ranges.
Exact performance of a point design can vary
within the range.
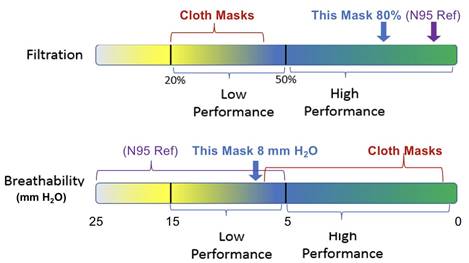
Erick shared the following thoughts “I have had
to emphasize with both Biden’s team as well as
the Aerosol Experts that in fact the standard is
a “Measurement Framework” that provides “ranges”
(20-50, 50-100). It does not specify performance
levels needed for any use case.
“We should adopt a different way of referring to
the ASTM standard going forward.: ASTM @
Filtration XX, Breathability YY, and FIT ZZ .
Why is this important ?
“There is already too much confusion regarding
mask assertions. The public message needs to be
concise, consistent, and comprehensible by the
general public. Social science research has
found that more than three elements that require
choice results in confusion.
In Europe there are tough ‘push” initiatives
with the requirement of N95 equivalent masks in
Germany, Austria, and France
ii
Pull - the advantage of using pull or incentives
1.
the safe bubble with the promise of full
occupancy within the bubble
We have identified SGS, Eurofins, MPR,
Mann-Hummel, Daikin, Johnson Controls, Ahlstrom,
Camfil, 3M, Honeywell, Cummins, Lydall, Berry,
and dozens of other companies who are capable of
quickly implementing the Pull portion of the
Push-Pull system.
ASTM labels and European Community mask
initiatives along with local and country
regulations provide the “push”. Part of the
push program is to limit occupancy of buildings.
If you limit occupancy to 25% whereas average
occupancy is 50% you have reduced the COVID risk
by half. The “pull” part of the program is to
offer validation for the actual risk
and alternative methods to reduce the risk to
much lower percentages.
In earlier Alerts we pointed out that most
facilities have many reasons to make visitors or
employees feel as safe as possible. If the risk
is reduced to the equivalent of 10% occupancy it
is five times lower than required.
The products and services are available to
implement the “pull” initiative immediately. The
challenge will be to create a rating system to
reflect the increased safety.
Eurofins has already done this for community
masks. We discussed their initiative on January
20. Here is their tested quality seal. Note
that by validating fit and comfort they are
making qualitative judgements. A validation of
risk reduction compared to 25% occupancy
involves a lesser qualitative component than one
on comfort.

One company with a number of applicable products
and services is SGS. One is a mobile monitoring
laboratory
“SGS is adding to our comprehensive COVID-19
Recovery Assistance Services by coming directly
to your location with our team, analytical
expertise and rental offerings. Our mobile
laboratory and field-deployable testing and
analytical equipment are now available for
SARS-CoV-2 detection in air and on surfaces.
SGS now offers near real-time Reverse
Transcriptase Quantitative Polymerase Chain
Reaction (RT-qPCR) data in air and on surface
swab sampling with our new mobile laboratory or
RT-qPCR instrument and analyst options. The
ability to provide near-real-time SARS-CoV-2
presence data using gold-standard RT-qPCR,
eliminates the 2-3-day turnaround time that was
needed to ship samples to the lab and wait for
results, a major step in speeding reaction to
possible COVID events. The service supports
analysis of air monitoring using Teflon air
filters and swab testing for cleaning
verification.”
LuxuryRes, in collaboration with SGS, has
developed and launched a new Hygiene Monitored
(HM) program to help hotels address the spread
of COVID-19. Here is how the user explains the
benefits.
“With a focus on selling, managing, and
marketing hotel properties with the use of its
own reservation and management technology,
LuxuryRes will now be able to provide cleaning
audits via both self and remote assessment as a
result of the HM program.
Hotels that successfully pass their audits will
be able to display the HM Mark, which not only
verifies their enhanced hygiene practices, but
also makes booking easier for business
travelers, as the HM mark enables them to
compare the hygiene levels of inspected hotels.
The HM program comes at a time when cleaning and
safety standards for travelers have become the
most important deciding factor for corporations
reviewing their business travel programs in
response to COVID-19. Hotels will be able to use
the HM program to validate the thoroughness of
their cleaning procedures and increase hotel
occupancy. The program includes a
dedicated addendum covering compliance with WHO
guidelines, which will help hotels to
demonstrate their commitment to mitigating the
spread of the virus.”
Note the claim is to “validate thoroughness of
cleaning and increase hotel occupancy” So this
is highly qualitative and clearly shows the
desire of the user to do something better than
the minimum.
SGS also has related services for building
owners including
Outbreak Detection
-
Emergency protocols activation
-
Crisis steering committee
-
Risk communication (internal and
external) activation
-
Initial pathogens monitoring and
baseline definition
With the pull approach, safe bubbles are created
and certified.
Those who achieve the certification can
operate at full capacity.
There is also the competitive pull.
The hotel, restaurant, or theater which
is viewed as safest will have a competitive
advantage
iii Criteria
1.
general and absolute
These criteria can include media penetration,
breathability and fit. The requirement to wear a
masks or to keep social distancing of six feet
are in this category. The requirement to use a
mask meeting the ASTM
20 or 50 standard is also absolute
2.
relative risk reduction as proposed by Mcilvaine
Mask risk is established in a five stage
sequence where steps 4 and 5 become very
flexible. Mask quality has to be addressed
starting with manufacturing and ending with the
final fit inspection.
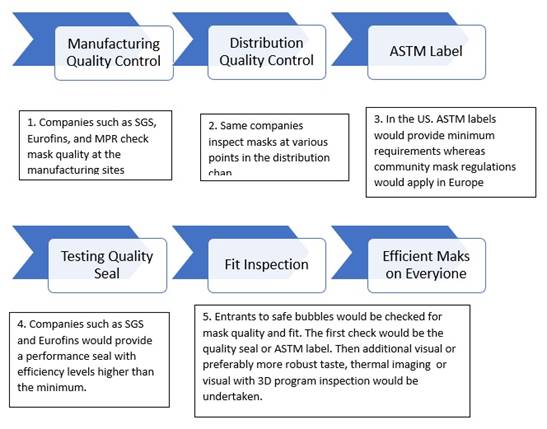
A very useful criterion will be the relative
risk. If the law sets a relative risk factor
equivalent to 25% occupancy by individuals in
cloth masks then alternatives can be evaluated
compared to this risk level.
If everyone (transmitters and recipients) were
wearing CATER 95 masks the net viral reduction
would be 99%. Because the virus can no longer
move from the transmitter to a surface the
overall risk is decreased by 98%. The most
efficient vaccine is only 95%. CATER masks can
quickly be made available. Facilities adopting
the CATER 95 Protocol could immediately open.
Industrial companies, corporate offices,
retailers, gyms and any facility where the
access is limited to CATER mask wearers should
be able to operate normally. Each employee or
visitor would have to wear an approved mask.
The CATER 95 is more than 90 times more
effective than the typical mask now being worn.
It is more than 70 times more effective than the
proposed ASTM 20 mask. A facility which is 100%
open with everyone wearing a CATER 95 mask will
be 25 times safer than a facility which is only
25% open but allows any type of mask to be worn.
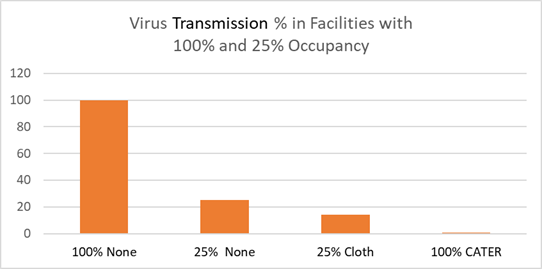
So a safe bubble with 100% occupancy but with
everyone wearing CATER 95 masks could be
certified to be as safe as required by the
25%/cloth mask risk level.
iv Product availability
1.
media
It is a
common belief that there is not enough efficient
media available. If we use procedural masks with
braces, this is not the case.
2.
masks
The availability of sufficient masks needs to be
communicated. The variety of acceptable mask
styles and the reuse are important aspects.
3.
timing
Determination has to be made and communicated as
to how rapidly everyone can be supplied with
suitable masks.
4.
geographies
Communication initially can be directed at the
U.S. followed by Europe and then the rest of the
developed world. As soon as possible it is also
important to communicate with the poorer
countries. New variants will be arriving in the
developed world from the developing world unless
the virus is eliminated everywhere.
Click here to register for the February 5
webinar:
https://home.mcilvainecompany.com/index.php?option=com_rsform&view=rsform&formId=92
RDI Medical has High Speed Mask Making Machinery
RDI Medical, a subsidiary of U.S. based company
The RDI Group, has focused on mask production
ever since the start of the coronavirus
pandemic. Having over a century of previous
experience in machine manufacturing and
automation, RDI Medical wasted no time in
producing these machines to supply the world
with this critical protective equipment.
RDI Medical has been designing and building
high-speed machines capable of producing masks
ranging from 1-ply to 4-ply, with printed logos
for businesses who want to incorporate their
brand. For businesses starting out in mask
production, RDI Medical helps specify raw
materials and recommendations to provide a
buying experience unmatched by other mask
machine manufacturers.
Curtis Maas, chairman and CEO of The RDI Group,
says: “The RDI Group supplies production systems
and technology to the best manufacturers in the
world—entering this PPE market for engineered
mask production was a logical decision for us.”
Capable of producing up to 200 masks per minute,
the face mask machinery produced by RDI Medical
provides manufacturers with standard and
customized equipment to keep up with the high
demand for face masks.
The RDI Group has over 100 years of established
engineering and automation experience and
applied their knowledge to the design and
manufacturing of this mask making equipment.
Additionally, RDI Medical is also offering high
performance and high-quality face masks for
commercial and institutional businesses as well
as wholesalers.
China Reports COVID Outbreak At Chicken
Processing Plant
China reported its first cluster of COVID-19
cases among workers in a meat processing plant,
raising fears among local consumers who have
until now mainly worried about the safety of
imported foods.
Ten confirmed cases were found in a factory
which slaughters 50 million chickens a year in
the northeastern city of Harbin and is owned by
Thai conglomerate Charoen Pokphand, one of the
world’s top poultry producers.
Another 28 workers at the plant and three family
members were asymptomatic, officials told a news
briefing on Thursday.
While China repeatedly pointed to imported
frozen meat and fish as the source of
coronavirus cases last year, it has not reported
significant clusters in its own food processing
sector.
Meatpacking workers in the United States, Brazil
and across Europe were among the groups hit
hardest by COVID-19 last year, with thousands of
slaughterhouse staff infected.
The cluster in the C.P. plant was detected as
part of routine screening of people in the
region, which has seen a surge in cases in
recent weeks.
Samples taken from inside the slaughterhouse,
its cold storage area and the outside of product
packaging during inspections earlier this week
had also been found positive for the virus, city
officials said.
The factory could not be reached for comment on
the outbreak. Officials at the company
headquarters in Bangkok made no immediate
comment.
