
Coronavirus
Technology Solutions
January 27, 2021
A Three-Step Strategy to Support the New U.S.
Mask Mandate
Let’s Organize a Webinar Around This Three Step
Mask Strategy
World Economy Could be Slowed by Lack of
Vaccines in Emerging Countries
Expert Cites Challenges in Vaccinating Poorer
Countries
Jiangsu Blue Sky is Supplying Three Layer FFP 2
Masks
Nanofiber Swabs are Better for Measuring COVID
CNN Perspective on the New ASTM Standards
_______________________________________________________________________________
A Three-Step Strategy to Support the New U.S.
Mask Mandate
This analysis just appeared in the Harvard Business Review and is by experts who we have quoted in the past (see authors at end of article).
Summary
- The Biden administration’s efforts to promote
the wearing of masks to combat the spread of
Covid-19 are badly needed. Given the pace of the
rollout of vaccines, the U.S. won’t achieve herd
immunity until mid-or late 2021. In the
meantime, mask wearing is essential.
We couldn’t agree more with the Biden
administration’s plea for
Americans to wear masks for 100 days and its
mandates that people must wear masks on federal
property and during interstate travel on
airlines, trains, buses, and ships. These
actions are crucial to address the surges in
Covid-19 cases and hospitalizations that
are occurring across the United States this
winter.
Although two effective Covid-19 vaccines are
being distributed in the United States and
others hopefully will be available soon, it will
probably take until sometime in
mid to late 2021 for enough people to be
vaccinated to reach herd immunity and for life
to start returning to normal in the United
States. Widespread vaccination is expected to
take multiple years globally.
In the meantime, with the growing concern about
Covid-19 mutations, CEOs, mayors, and governors
should immediately take three steps to promote
the effective use of face masks.
1. Launch an awareness blitz. To
increase the utilization of masks and dispel
misinformation, an effective campaign is needed
to raise awareness of the mask recommendations.
With few exceptions, all people should wear
masks consistently when sharing airspace with
others from outside their
bubble of family, friends, and coworkers. They
are not currently doing so.
In a U.S. national survey we conducted in
December, over 85% of the 466 respondents said
they were using masks to protect themselves and
those around them (consistent with a similar
survey by
Pew in August), and 80% said they were using
their masks at the grocery store. But only 56%
said they used one when with people outside
their home, only 48% were doing so at work, and
just 33% were wearing them when someone visited
their homes.
The usage
of masks and
the way they are being worn also varies from
city to city: Using publicly accessible street
cameras, we recently counted how
many people were correctly wearing masks in one
location in San Francisco (Castro
Street)
and another in Los Angeles (Hermosa
Beach);
while 90% of people in the former were correctly
wearing masks, only 60% in the latter were doing
so.
2. Communicate which mask types people should
wear. In
our survey, the majority (71%) said they were
using basic masks (cloth or surgical masks),
which are a reasonable option for people at low
risk of contracting a severe case of Covid-19
and have limited exposure to the virus or to
people outside their bubble. Regions in Kansas and Germany that
required basic (any) masks had much lower rates
of infection than regions that did not.
But people at higher risk of contracting a
severe case of Covid-19 or who have exposure to
others outside their small bubble may require a higher-filtration
mask.
For example, wearing a basic mask did not stop
infections altogether on a long-haul flight in Boeing
777 equipped
with HEPA filtration and among workers at a seafood-processing
plant and meat
processing plants.
Surgical masks distributed on an Argentine
cruise ship during
an outbreak and in a Danish
randomized controlled study did
not prevent transmission altogether, although
these masks may have reduced the severity of
symptoms. Germany, France, and other European
countries are now requiring high-filtration
(medical) masks in public.
New standards being developed by
ASTM International, an international standards
organization, for labels that display the
filtration efficiency of face masks for
consumers are coming in the near future. Several
options for high-filtration masks are
considerably more effective in limiting the
spread of Covid-19 than basic cloth masks or
consumer-grade “surgical” masks.
A few simple ways to improve masks’ fit and
filtration for the general public that have
been recently
studied include
putting a high-quality cloth mask on top of a
surgical mask or sandwiching a surgical mask (or
higher-quality filter) in-between two cloth
masks. Consumer-grade surgical masks can be upgraded with “fitter” add-ons
like Fix-the-mask to
improve their fit, thereby enhancing the
filtration of viral particles.
The N95 respirator is the best-known
high-filtration mask in the United States.
(Comparable models in other parts of the world
include FFP2 in
Europe, KF94 in
South Korea, and KN95 in
China). In our survey, 13% of respondents
reported that they were wearing an N95 or the
equivalent. In a Finnish
study of
health care workers, no infections occurred at
work while wearing N95 type respirators, but 63%
of workplace infections occurred while wearing
surgical masks.
Disposable N95s,
which have been in short supply during the
pandemic, have been largely reserved for health
care workers. But N95s are now available at Costco, Amazon, Office
Depot,
and some manufacturers point out that demand for
N95s from the general public will help even out
the ebbs and flows of demand from hospitals,
allowing them to maintain
consistent production.
To be effective, N95s also need to be properly
fitted,
and users need to be trained to wear them
correctly. While in a setting like a hospital, a
respiratory protection program can ensure that
this happens, that’s not feasible for the
general public.
As we wrote in October,
a U.S.-manufactured, federally-approved option that
is not in short supply and
is reusable is an elastomeric N95 (eN95)
respirator. Since that article was published, a
number of organizations — most notably the Fire
Department of New York —
have begun to use them. In our survey, 9% of
respondents reported that they were wearing eN95
masks.
According to the CDC,
eN95s have sealing surfaces and adjustable
straps that can help achieve a better fit (or
lower leakage), and the replaceable filters in
some models can be used for one year as long as
the filter cartridges remain in good condition.
To protect others, many eN95 models also require a workaround to
cover their exhalation valve, although the CDC
recently reported the
maximum particle emissions through the valve are
similar to or better than surgical masks or
unregulated face coverings. Some manufacturers
(e.g., Envomask and MSA)
now address this by completely plugging the
valve. In addition, valveless, bidirectional,
and transparent high-filtration
masks designed for public use are also becoming
available. As with disposable N95s, fitting and
training are essential to ensure that workers
get the best protection possible.
Some people with asthma, chronic lung diseases,
or heart diseases may not be able to tolerate
N95 or eN95 respirators and should consult their
medical provider before using one. But for the
general public, we expect that wide availability
of respirators and low-leakage, high-filtration
face masks combined with education on how to use
them will significantly reduce exposure to the
coronavirus that causes Covid-19.
People might want to keep eN95s on hand even
after the pandemic ends for new outbreaks of
diseases that spread through the air, intentional
bio attacks,
and wildfire emergencies.
3. Prioritize the distribution of
high-filtration masks to the vulnerable. In
addition to upgrading indoor
ventilation and air filtration at places where
essential workers, older adults, and people with
comorbidities live or work, organizations should
make providing these populations with
high-filtration, low-leakage masks a top
priority. They need to be equitably subsidized
or provided for free to
people who cannot afford them.
In October we proposed providing federal
credits to
consumers to buy high-filtration masks, and
Germany is now sending
“vouchers” to
all its senior citizens over 60 years old and
vulnerable populations that can be redeemed for
12 FFP2 masks (N95-equivalents) at pharmacies
and grocery stores. That’s 34 million people. Austria implemented
a similar policy.
President Biden has requested Congress to
appropriate $30 billion for personal protective
equipment and signed the Defense Production Act
to boost production of masks. However, until
those resources become available,
it is up to CEOs, mayors,
and governors to implement these three steps to
ensure consistent use of the best possible masks
for vulnerable groups. These steps will
dramatically reduce the spread of Covid-19 and
save lives.
Authors of this article are
-
Devabhaktuni Srikrishna is
the founder of Patient
Knowhow,
which curates patient
educational content on YouTube.
In 2014, he worked on the
response to the Ebola outbreak
in Guinea. Follow him on Twitter
at @sri_srikrishna.
-
Joseph Buccina is
a director at In-Q-Tel’s B.Next,
a strategic initiative focused
on biotechnology and national
security.
-
Dan Hanfling,
MD, is a clinical professor of
emergency medicine at George
Washington University, co-chair
of the National Academy of
Medicine Forum on Medical and
Public Health Preparedness, and
a vice president on the
technical staff at In-Q-Tel.
-
Monica Gandhi,
MD, is an infectious disease
specialist, professor of
medicine, medical director of
the Ward 86 HIV Clinic, and
director of the Center for AIDS
Research at the University of
California, San Francisco.
Follow her on Twitter at @MonicaGandhi9.
-
Donald Milton,
MD, is a professor of
environmental and occupational
health at the University of
Maryland School of Public
Health. Follow him on Twitter
at @Don_Milton.
Let’s Organize a Webinar Around This Three Step
Mask Strategy
As Sri and the other authors point out the three
step plan needs to be quickly implemented and
“it is up to CEOs, mayors, and governors to
implement these three steps to ensure consistent
use of the best possible masks for vulnerable
groups.
Let’s arrange a webinar to discuss the
three step plan. Here is a proposed agenda of
subjects to be addressed.
The three steps are (l) launching an awareness
blitz, (2) advise on which masks should be worn
and (3) prioritize masks for the vulnerable
1.
Launch an awareness blitz
a.
roles
i.
mainstream media including local news outlets
ii.
role of association and occupational media
including business
and medical
iii.
suppliers from the large validators such as SGS
and Eurofin to media suppliers to mask
manufacturers
iv.
CATER Mask Decisions
b.
message
i.
push - the need to mandate use of efficient
masks
ii.
pull - the advantage of using pull or incentives
1.
the safe bubble with the promise of full
occupancy within
the bubble
iii.
criteria
1.
general and absolute
2.
relative risk reduction as proposed by Mcilvaine
iv.
product availability
1.
media
2.
masks
3.
timing
4.
geographies
2.
Communicate which type of masks people should
wear
a.
N95
b.
CATER
c.
surgical mask with brace
d.
eN95
e.
other
3.
Prioritize masks for the vulnerable
a.
who
b.
location – should this extend to other
countries?
c.
how
i.
vouchers
ii.
direct distribution
We will be arranging a meeting time. If you
would like to participate please communicate
with us and also add your thoughts on additional
aspects to address.
World Economy Could be Slowed by Lack of
Vaccines in Emerging Countries
With several Covid-19 vaccine candidates showing
promising trial outcomes,
investors and
analysts have turned increasingly optimistic
that the pandemic could soon come to an end.
But a new report by Citi Research showed that
the economic benefits of vaccination may not
kick in until late 2021, when “herd immunity” is
expected to start forming. Herd immunity occurs
when enough people in a population develop
protection against a disease that it can no
longer spread easily among them.
The report, written by Citi economists, drew on
a paper in the American Journal of Preventive
Medicine that simulated the percentage drop
in daily Covid-19 cases under various scenarios
of vaccine efficacy and coverage.
The paper, Vaccine
Efficacy Needed for a COVID-19 Coronavirus
Vaccine to Prevent or Stop an Epidemic as the
Sole Intervention,
concluded that vaccines must have an efficacy of
at least 70% to prevent an epidemic and at least
80% to “largely extinguish” an epidemic without
any other measures.
Among the current vaccine frontrunners, Pfizer-BioNTech
and Moderna reported preliminary results showing
that their respective candidates were around 95%
effective. Meanwhile, Oxford-AstraZeneca said an
interim analysis showed their vaccine having an
average efficacy of 70% in protecting against
the virus.
The Citi analysis assumes those three vaccine
candidates would receive emergency approvals
between next month and January 2021 — which
would allow the pharmaceutical companies to
produce and distribute their vaccines.
The economists said that developed economies,
many of which have secured vaccine pre-orders,
will first experience the economic benefits of
herd immunity.
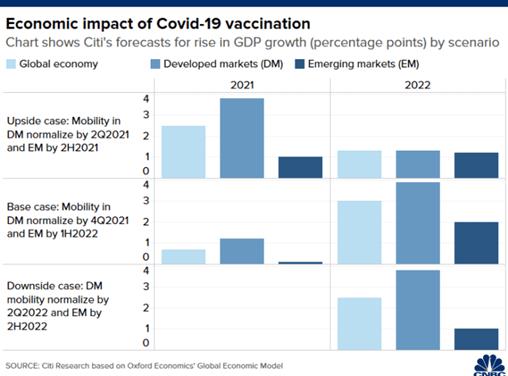
Overall, the bank has forecast that Covid-19
vaccination could raise global economic growth
by 0.7 percentage points in 2021, and 3
percentage points in 2022 as activity returns to
normal.
According to Citi, developed markets have
collectively secured 85% of total bilateral
pre-orders of Covid-19 vaccines. Countries such
as the U.S., U.K., Japan, Canada, Australia and
those in the European
Union have ordered
supplies that exceed their populations, the
analysts added.
That means major developed economies could start
wider distribution of the vaccines in the second
or third quarter next year, and form herd
immunity by the final three months of 2021, they
explained.
“Most people, who seek vaccination, may be
vaccinated at least by the end of 2021,” said
the analysts.
Normalizing economic activity is projected to
raise growth in developed markets by 1.2
percentage points in 2021 and 3.9 percentage
points in 2022, said Citi.
In comparison, emerging markets could see
growth increase by 0.1 percentage points in 2021
and by 2 percentage points in 2022, projected
the bank.
The smaller economic benefits in emerging
markets are partly because some countries,
especially those in Asia such as China, have
contained the virus and allowed most activity to
resume, Citi explained. In addition, vaccine
distribution may take a longer time to reach
emerging economies, with lower-income countries
potentially having to wait until end-2022 or
even later, the bank added.
Emerging markets may have to rely on the Covax
facility for vaccine supply, Citi analysts said,
referring to the United
Nations’ program that will subsidize rollouts of
Covid-19 vaccines to
low-income countries.
Citi said there are uncertainties that could
alter the timing of its forecasts for when herd
immunity will be reached.
Those factors include:
-
Efficacy of vaccines and
Covid-19 reproduction rate,
which refers to the number of
people that an infected
individual goes on to infect.
-
The speed at which mass
production of vaccines can be
ramped up.
-
People’s acceptance to a
vaccine.
The bank, citing a survey
by Ipsos and the World Economic Forum,
pointed out that
vaccine acceptance appeared to have fallen in
recent months. The survey conducted in October
found that 73% of respondents across 15
economies intend to get vaccinated — 4
percentage points fewer than the same survey
done three months earlier.
“In general, vaccine coverage should reach at
least 70% to form some herd immunity,” said Citi.
“However, vaccine acceptance rates of 54%-59% in
France, Hungary, Poland and Russia suggest
potential delays in the timing of herd immunity
by vaccination in some countries.”
Expert Cites Challenges in Vaccinating Poorer
Countries
The global vaccine rollout is full of glitches,
shortages, and problems, but not every country
faces the same challenges. Evening out those
inequalities to make sure poorer countries are
included in the vaccination race isn’t just the
ethical thing to do it’s good for rich
countries, too. A new study from the National
Bureau of Economic Research shows that the entire
global economy depends on poorer countries’
getting residents vaccinated:
advanced economies
will still bear 49% of the costs of the
pandemic, even if they get their own populations
entirely inoculated.
With a new leader in the White House, we’re
seeing signs that the US will do its part. The
Biden administration says it will join Covax, a
global vaccination effort led by the World
Health Organization that aims to get
the first batches of vaccines to poorer
countries in
February. To learn
more about global vaccine inequality, MIT
Review spoke with Anita Ho, associate
professor in bioethics and health services
research at University of British Columbia and
the University of California, San Francisco.
This interview has been condensed and edited for
clarity.
Q: What’s the upshot of the US joining Covax? Do
you expect that to be any sort of game-changer
for global vaccine inequity?
A: Even from a symbolic perspective it’s
really important to have the US rejoining the
WHO efforts and Covax. It’s also important for
financial reasons, because Covax needs money to
get supplies. It’s not just vaccines ... we need
money for personnel, we need money for
protective equipment. We need money for glass,
for syringes, for needles—everything. So the US
being there can provide leadership and provide
more financial security as well.
Q: So it really goes beyond just needing the
doses. What are some of the biggest disparities
in global vaccine distribution right now? It
sounds like supplies might play into that.
A: It’s not just Are
we willing to donate the vaccines? It’s Do
we have the infrastructure to even store and
transport the vaccines? The main ones
approved in the US, for example—the Pfizer and
Moderna vaccines—really require very cold
refrigeration. That is not even very feasible in
some areas of the world that have limited
electricity supply.
Despite early gains, Israel’s vaccine rollout is
still leaving far too many people out, says one
expert.
One of the greatest contributors to the
disparity is when wealthier nations pre-order
vaccines from manufacturers. They’re buying up
most of the supply—the potential supply, even.
So even when companies are ramping up the
supply, they’re not going to be able to go to
these poorer countries unless Covax can buy
them.
Q: You’ve spoken
before about vaccine
disparities even within high-income countries.
Why do those happen? What should we be watching
out for?
A: Think about how people get notifications that
it’s their turn to get vaccinated. In the US,
for example, the ones who’d get those
notifications would have smartphones, have email
addresses, would already have a primary care
provider. If you are undocumented, if you are
homeless, you may not have access to that
information and you wouldn’t even know.
The Pfizer, Moderna, and AstraZeneca vaccines
all require two doses with variable time between
them. This means we need careful tracking of
individuals twice: to get their first dose, and
then to get them to return at the right time
frame to get their second dose. For people in
remote areas, or places that don’t have
convenient access to pharmacies—often poorer
neighborhoods—it’s difficult for them to travel
twice. And for people who may be homeless or
without cell phones, it would be challenging to
reach them twice in a designated time frame. So
one way to promote vaccination equity is to have
reserve vaccines that would only need one dose
for these populations. Johnson & Johnson is
developing a one-dose vaccine right now.
There may also be another disparity. There are
many people who, even if you offer them the
vaccine, will not take it. And that’s partly
because of the distrust. There is a much higher
level of distrust among Latino and Black
Americans, partly because of historical
mistreatment.
Q: How are you seeing mistrust affect global
vaccination disparities more globally?
A: When we think about mistrust on a global
scale, that may be partly because of how the
pharmaceutical industry prices things and how
they have patents. Some countries may be
thinking, “These companies from the US or Europe
are really trying to sell us their expensive
vaccines. But we can’t really afford them for
our population in the first place because they
are patented, and we are not allowed to just
make a generic version of it.” They may be
thinking, “These companies are just trying to
take advantage of us.” And there certainly have
been examples of lower-income countries that
have been exploited by the pharmaceutical
industry.
Inequitable vaccine allocation definitely will
disrupt the supply chain for all, including the
wealthiest nations that have come to depend on
cheap sources of labor.
In Indonesia, for example, this happened with
H5N1. Whenever there’s an outbreak, if you’re a
WHO member, you send samples to a WHO lab and
they try to find out about this particular virus
or disease. Based on genetic material sent from
Indonesia, scientists developed therapeutics for
H5N1 and tried to sell them back to Indonesia.
Then Indonesia thought, “Okay, these were our
samples. Should there not have been
collaboration? You’re using them to sell drugs
back to us.”
Q: Does the US have a moral obligation to send
people to other countries to help with
vaccinations?
A: One of the problems is that we’re not able to
train enough people in the local places. For
Covax or other kinds of international
collaboration, it’s not about sending people so
much as it’s about how do we help them build up
their own infrastructure? Even financial
resources for training courses or other kinds of
ways to beef up their own human resources.
Because you can imagine we’d go, and then we’d
leave, and they’re not any better in terms of
infrastructure.
Q: How would it affect higher-income countries
if other, lower-income countries don’t receive
their vaccines until later? Recent research
says, for example, that if poor countries don’t
get vaccines, it
will disrupt the economy for everyone.
A: While it’s still likely that at the human
level, people in the most vulnerable countries
will suffer more, inequitable vaccine allocation
definitely will disrupt the supply chain for
all, including—perhaps even especially—the
wealthiest nations that have come to depend on
cheap sources of labor. If supplying nations
have lots of people being sick, or they have to
shut down, [there are] no workers to process or
transport the raw materials, or to manufacture
and deliver the products. People in these
countries also can’t travel or spend money,
which can greatly affect international hotel
chains, airlines, and hospitality industries as
well.
This would apply within a high-income country
too. If undocumented workers, farm workers,
homeless people, and others in low-wage jobs
can’t get vaccinated, they can’t work to keep
the supply chain going. So restaurants,
entertainment industries, etc. would suffer. If
they can’t pay the rent or mortgage or have
extra money, that also affects the rest of the
economy.
Jiangsu Blue Sky is Supplying Three Layer FFP 2
Masks
This Chinese company is a major international
exporter of dust collector media and bags. They
now are supplying disposable masks.
Edward Wu wrote us with the following,
“This year we are also to produce the FPP2,
FFPP1, KN95, paper diaper, etc. use ES hot air
cotton, SS, SMS, etc. fabric, the ES hot air
cotton machine is imported from Taiwan, could do
the weight from 20-50 gsm, width below 3m such
fabric for you, each day could do about 8-12
Ton.


Nanofiber Swabs are Better for Measuring COVID
Following the COVID-19 outbreak, swabs for
biological specimen collection were thrust to
the forefront of healthcare materials. Swab
sample collection and recovery are vital for
reducing false negative diagnostic tests, early
detection of pathogens, and harvesting DNA from
limited biological samples.
In this study (linked below), we report a new
class of nanofiber swabs tipped with
hierarchical 3D nanofiber objects produced by
expanding electrospun membranes with a
solids-of-revolution-inspired gas foaming
technique. Nanofiber swabs significantly improve
absorption and release of proteins, cells,
bacteria, DNA, and viruses from solutions and
surfaces.
Implementation of nanofiber swabs in SARS-CoV-2
detection reduces the false negative rates at
two viral concentrations and identifies
SARS-CoV-2 at a 10× lower viral concentration
compared to flocked and cotton swabs. The
nanofiber swabs show great promise in improving
test sensitivity, potentially leading to timely
and accurate diagnosis of many diseases.
https://pubs.acs.org/doi/full/10.1021/acs.nanolett.0c04956#
CNN Perspective on the New ASTM Standards
The three step plan discussed earlier includes
activities to inform the main stream media about
mask options.
Here is the CNN reporting this week.
A draft of the first national mask evaluation
standard for consumer masks obtained by CNN
shows proposed guidance would call for two tiers
of certification.
-
A level one mask would
require the product to filter
20% of particles -- something
that would make the mask easy to
breathe through, but that would
provide minimal protection.
-
A level two mask would
require "high performance"
filtration of at least 50% of
particles but would provide less
breathability.
The standards are currently in development with
ASTM International and the National Personal
Protective Technology Laboratory, which is an
arm of the US Centers for Disease Control and
Prevention’s National Institute for Occupational
Safety and Health.
The current standards: Currently,
only medical-grade masks and respirators must
meet standards. These include N95 masks, which
are regulated for fit, filtration efficiency,
flammability and other qualities.
The new standards: The
proposed standards will outline specific fit,
design, performance and testing requirements for
face masks and coverings, according to a draft
of the standards provided to CNN by ASTM
International.
The draft evaluates both single use and reusable
masks and outlines specific requirements. For
instance, the standards would prohibit the use
of vents, valves or any feature that allows air
flow to bypass filtration -- though there are
exceptions to this that reflect current CDC
guidance.
The review process is ongoing, and these
guidelines are subject to further review and
change. The drafted guidelines will be further
reviewed next week.
The ASTM draft standard currently is far
different from standards required for masks in
several European countries. Germany, Austria and
France are now requiring people wear masks with
a minimum filtration efficacy of 80-90% while on
public transport, shopping or in public areas.
The overall takeaway from reading this article
is that the U.S. will be falling behind
European countries even with the ASTM
standard.
The article also does not emphasize that the
ASTM
standard is not a regulation whereas the
European standards are required to be met. It
also implies that these standards have the same
weight as the current standards which are
enforceable in medical applications.
So there is no real distinction between
creating a standard and writing regulations to
enforce it .
