
Coronavirus
Technology Solutions
January 4, 2021
Vaccines are not
a Short Term
Solution so We
Need the Safe
Bubble
Initiative
New Methods are
Needed
to Assure
a Tight Fit on
Every Mask Worn
in Public
Suppliers of
Disposable Masks
are Focused on
Increasing
Production and
Not Necessarily
Holistic
Solutions
Important New
Findings on
Performance
Differences
Between Masks
N95 Tested at
98% FFE
_____________________________________________________________________________
Vaccines are not
a Short Term
Solution so We
Need the Safe
Bubble
Initiative
There are many
reasons why we
will need to
depend on HVAC
and masks as
part of a Safe
Bubble
Initiative (SBI)
for at least
several years.
·
Hundreds of
thousands of
wealthy citizens
of the world
will die in the
next 12 months
without the SBI.
·
Millions of
poorer citizens
will die in the
next 4 years
without SBI.
·
Major impact of
the vaccines
will not be felt
in wealthy
countries until
late in the year
and not for
years in poorer
countries.
·
New variants of
the virus are
eventually
likely to evolve
into one which
resists the
present
vaccines.
·
Prevention of
future pandemics
along with
efforts to
reduce the
impact of air
pollution,
wildfires, and
influenza will
provide long
lasting
benefits.
New Methods are
Needed
to Assure
a Tight Fit on
Every Mask Worn
in Public
Mask leakage can
vary from 2 to
60%.
The
differences in
virus
transmission
are huge
when you include
both emitter and
recipient. At 2%
escaping from
the transmitter
only 2 x .02 or
.04%
of the
air leaks into
the recipient
mask. With the
60% leakage 36%
can be
inhaled by the
recipient. So
36/04 or 900
times more virus
is inhaled with
the looser
masks.
When you combine
air leakage and
media efficiency
for both
transmitter and
recipient the
same high ratios
are found.
However, other
measures such as
limiting
capacity are
less effective
and hugely
costly to the
economy. You can
require a school
to operate at
10% capacity but
that only
provides a 10 to
1
reduction
ratio.
With the Safe
Bubble
Initiative every
entrant to a
facility would
be checked to
assure that his
mask is tight
fitting and
efficient. There
would be a
tiered approach.
· Tier One: Every mask type and size would be tested by the manufacturer under various motions and with individuals whose facial features match the mask size. Quantitative fit testing would be used. An approved fit testing laboratory would conduct these tests and provide the rating. The entrant to a facility would only have to show that he is wearing a rated mask and has chosen a proper size.
·
Tier One
Alternative:
Local operators
such as Fitness
Centers or
Department
Stores could
sell masks and
provide fit
testing for each
purchaser. If
they are selling
one brand of
mask with five
sizes, they
select the
appropriate size
for the
purchaser and
run the two
minute fit test.
·
Tier Two: Each
entrant would be
checked to see
that he has an
accredited mask
and that it is
being worn
properly. This
inspection could
include
something as
elaborate as
periodic
qualitative
testing or just
visual
inspection.
One or more
visual methods
need to be
created. A
visual check can
determine if the
mask has the
following
attributes:
·
Stays in
position on face
across a variety
of motions:
walk, talk, bend
over, head side
to side
·
Does not
restrict field
of vision
·
Adjustable
noseband to seal
gaps on either
side of
nosebridge-mandatory
·
Head strap
accessory for
alternate
attachment with
adjustable
tension in back
of head-optional
·
Trim or other
design element
to create a seal
between user's
face and
mask-optional
The manufacturer
also should
supply
·
Correct donning,
doffing, and
noseband
instructions
·
Product support
to ensure
correct size
·
Offer in several
sizes to fit a
wide range of
facial shapes
and structures
·
Practical
performance
testing on test
subjects to
determine
leakage under
normal
activities (on
sample test
subjects in lab
setting)
A manual check
can also be
provided. Here
is the procedure
recommended by
Vogmask.
“Place your fingers
on the
cheekbones and
thumbs on sides
of chin to do
inhale and
exhale fitting
check.
"Inhale slowly.
Check to see if
the facepiece
suctions
slightly towards
your face. No
air should leak
in between your
face and mask.
- Exhale slowly. The facepiece should be bulging slightly outwards as exhale exits back through facepiece.
- Check again for leaks between your face and the facepiece of the respirator.
- If you detect any leaks, readjust the ear loops or head straps and check again for fit.
- If you cannot get a good seal around nose and mouth, the mask is not correct size and we request you contact us.”
Additional ways
to check the fit
could be
developed.
Some
creativity is
needed. Here is
one idea.
Instead of
lighting up a
pumpkin why not
use a cheap
disposable light
to light up the
openings in the
mask.
the
subject would
place the light
in his mask. He
could manipulate
it through the
soft fabric or
with attached
threads and
change the
trajectory of
the light beam
to check the
periphery
while the
inspector is
watching.
Lights for
lanterns are 30
cents each on
Amazon. So they
could be given
to the entrant
after use.

Variations of
qualitative
testing could be
performed. A
test only
requires a hood
and method of
injecting a
sweet or bitter
aerosol.
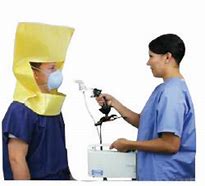
Many facilities
have
some sort
of
a line
where
temperature is
checked.
They
could be
utilized with a
walk through
unit. A fan
filter unit and
walk in module
would cost less
than $5k.
Each
entrant could be
tested with a
modified taste
test.


These are just a
few approaches.
There could be
much better ones
if only a little
time is spent
devising them.
When you
consider that
the mask fit is
as important as
vaccines and
more important
than social
distancing it is
important to
measure it and
act accordingly.
Suppliers of
Disposable Masks
are Focused on
Increasing
Production and
Not Necessarily
Holistic
Solutions
The huge
production
requirements
based on
multiple mask
use per day for
healthcare
workers will
hamper efforts
to supply enough
masks and filter
media to
properly address
COVID. Therefore
reusable masks
are a critical
necessity.
3M
replied
to the McIlvaine
proposed
Safe
Bubble
Initiative by
pointing out
that all their
resources are
needed to make
disposable masks
for healthcare
workers. At the
very least there
should be an
effort to see
how many times
disposable N95
masks can be
worn by the
public before
the fit and
efficiency
deteriorate.
Many of the two
billion masks 3M
hopes to make
will be needed
for
applications
where a
disposable mask
is most
appropriate.
When a nurse is
entering a COVID
ICU unit and
then may be
entering the
room of a cancer
patient the risk
of transmission
from one patient
to another is
too high to
consider wearing
a previously
used and
sterilized mask.
But the average
individual who
has probably not
been exposed to
COVID during the
day can wear his
mask multiple
times. The N95
mask can have a
high net
efficiency and
even with some
deterioration
will
be many
times more
effective than
the typical
cloth
mask.
The 3M position
is
understandable.
They are to be
commended for
the great effort
they have made
to increase
production. It
may turn out
that there
really is a need
for the two
billion masks
they make to be
used by medical
personnel. If
four masks are
required each
day then this is
only enough for
500 million mask
days or 1.7
million people
for 300 days.
Here is
the 3M reply.
Thank you for
your inquiry. 3M
is committed to
supporting
public health
and the
government
response to the
COVID-19
outbreak. While
3M appreciates
offers to
collaborate, we
are unable to
commit resources
to review your
new product idea
at this time.
Since the
outbreak of
COVID-19, we
have increased
our production
of personal
protective
equipment, and
we are working
to nearly double
our production
of respirators,
to almost 2
billion
globally, within
the next 12
months. A
diversion of
resources would
reduce our
ability to
maximize
production of
critical
supplies and
therefore reduce
our ability to
have the
greatest public
health impact
possible.
While we are
unable to work
together at this
time, we may be
interested in a
potential
collaboration in
the future when
our production
schedules are
back to normal
volume. You are
welcome to
submit your
unsolicited,
non-confidential
idea at
https://www.3m.com/3M/en_US/company-us/unsolicited-idea-submission-policy/.
That link will
provide
guidelines for
3M acceptance of
unsolicited
ideas. As a
reminder, you
are free to
submit your
ideas to other
companies.
Thank you for
your
understanding as
we work to
deliver high
volume
production in
the short to
medium term.
We are grateful
for your
commitment to
public safety
during the
rapidly changing
COVID-19
outbreak and are
amazed by the
number of people
and companies
willing to come
together to help
protect our
healthcare
workers on the
front lines of
this fight.
Important New
Findings on
Performance
Differences
Between Masks
A new study for
CDC confirms
many past
studies. Tight
fitting and
efficient masks
perform
much better than
the average
public mask. An
abstract and
summary is
provided below
along with a
link to the full
article.
The
article provides
Evaluation of
Cloth Masks and
Modified
Procedure Masks
as Personal
Protective
Equipment for
the Public
During the
COVID-19
Pandemic
Phillip
W. Clapp, PhD1,2; Emily
E. Sickbert-Bennett, PhD,
MS3; James
M. Samet, PhD,
MPH4; et
alJon Berntsen, PhD5; Kirby
L. Zeman, PhD2; Deverick
J. Anderson, MD,
MPH6; David
J. Weber, MD,
MPH3,7; William
D. Bennett, PhD2,7; for
the US Centers
for Disease
Control and
Prevention
Epicenters
Program
Key Points
Question: What
are the fitted
filtration
efficiencies (FFEs)
of
consumer-grade
masks,
improvised face
coverings, and
modified
procedure masks
commonly used
during the
coronavirus
disease 2019
(COVID-19)
pandemic?
Findings: In
this comparative
study of face
covering FFEs,
we observed that
consumer-grade
masks and
improvised face
coverings varied
widely, ranging
from 26.5% to
79.0% FFE.
Modifications
intended to
enhance the fit
of medical
procedure masks
improved FFE
measurements
from 38.5%
(unmodified
mask) to as much
as 80.2%.
Meaning:
Simple
modifications
can improve the
fit and
filtration
efficiency of
medical
procedure masks;
however, the
practical
effectiveness of
consumer-grade
masks available
to the public
is, in many
cases,
comparable with
or better than
their non-N95
respirator
medical mask
counterparts.
Abstract
Importance:
During the
coronavirus
disease 2019
(COVID-19)
pandemic, the
general public
has been advised
to wear masks or
improvised face
coverings to
limit
transmission of
severe acute
respiratory
syndrome
coronavirus 2
(SARS-CoV-2).
However, there
has been
considerable
confusion and
disagreement
regarding the
degree to which
masks protect
the wearer from
airborne
particles.
Objectives:
To evaluate the
fitted
filtration
efficiency (FFE)
of various
consumer-grade
and improvised
face masks, as
well as several
popular
modifications of
medical
procedure masks
that are
intended to
improve mask fit
or comfort.
Design, Setting,
and Participants:
For this study
conducted in a
research
laboratory
between June and
August 2020, 7
consumer-grade
masks and 5
medical
procedure mask
modifications
were fitted on
an adult male
volunteer, and
FFE measurements
were collected
during a series
of repeated
movements of the
torso, head, and
facial muscles
as outlined by
the US
Occupational
Safety and
Health
Administration
Quantitative Fit
Testing
Protocol. The
consumer-grade
masks tested
included (1) a
2-layer woven
nylon mask with
ear loops that
was tested with
an optional
aluminum nose
bridge and
nonwoven filter
insert in place,
(2) a cotton
bandana folded
diagonally once
(i.e., “bandit”
style) or in a
(3) multilayer
rectangle
according to the
instructions
presented by the
US Surgeon
General, (4) a
single-layer
woven
polyester/nylon
mask with ties,
(5) a nonwoven
polypropylene
mask with fixed
ear loops, (6) a
single-layer
woven polyester
gaiter/neck
cover balaclava
bandana, and (7)
a 3-layer woven
cotton mask with
ear loops.
Medical
procedure mask
modifications
included (1)
tying the mask’s
ear loops and
tucking in the
side pleats, (2)
fastening ear
loops behind the
head with
3-dimensional–printed
ear guards, (3)
fastening ear
loops behind the
head with a
claw-type hair
clip, (4)
enhancing the
mask/face seal
with rubber
bands over the
mask, and (5)
enhancing the
mask/face seal
with a band of
nylon hosiery
over the fitted
mask.
Main Outcomes
and Measures: The
primary study
outcome was the
measured FFE of
common
consumer-grade
and improvised
face masks, as
well as several
popular
modifications of
medical
procedure masks.
Results: The
mean (SD) FFE of
consumer grade
masks tested on
1 adult male
with no beard
ranged from
79.0% (4.3%) to
26.5% (10.5%),
with the 2-layer
woven nylon mask
having the
highest FFE.
Unmodified
medical
procedure masks
with ear loops
had a mean (SD)
FFE of 38.5%
(11.2%). All
modifications
evaluated in
this study
increased
procedure mask
FFE (range [SD],
60.3% [11.1%] to
80.2% [3.1%]),
with a nylon
hosiery sleeve
placed over the
procedure mask
producing the
greatest
improvement.
Conclusions and
Relevance: While
modifications to
improve medical
procedure mask
fit can enhance
the filtering
capability and
reduce
inhalation of
airborne
particles, this
study
demonstrates
that the FFEs of
consumer-grade
masks available
to the public
are, in many
cases, nearly
equivalent to or
better than
their non-N95
respirator
medical mask
counterparts.
Introduction
Severe acute
respiratory
syndrome
coronavirus 2
(SARS-CoV-2),
the cause of
coronavirus
disease 2019
(COVID-19), is a
transmissible
virus that
infects the
upper and lower
respiratory
tract, leading
to a high viral
titer in saliva
and respiratory
secretions. A
key public
health control
strategy for
mitigating
SARS-CoV-2
transmission is
use of masks or
face coverings
by the
public. Masks
that completely
cover the nose
and mouth are
effective at
reducing
seasonal
coronavirus and
influenza
transmission
when worn by
infected
persons and
noninfected
persons who may
come into
contact with
infected
individuals. This
is supported by
emerging
epidemiologic
data that
indicate that
community-wide
use of masks can
effectively
contribute to
the prevention
of SARS-CoV-2
transmission.
As the adoption
of face
coverings during
the COVID-19
pandemic becomes
commonplace,
there has been a
rapid expansion
in the public
use of
commercial,
homemade, and
improvised masks
that vary
considerably in
design,
material, and
construction.
Similarly, the
press and social
media outlets
have reported on
numerous
innovative
“hacks,”
devices, and
modifications
(enhancements)
that claim to
improve the
performance
characteristics
of conventional
masks (typically
surgical or
procedure
masks). Despite
their widespread
dissemination
and use during
the pandemic,
there have been
few evaluations
of the
efficiency of
these face
coverings or
mask
enhancements at
filtering
airborne
particles. In
this study, we
used a recently
described
methodological
approach based
on the
Occupational
Safety and
Health
Administration
(OSHA) Fit Test
to determine the
fitted
filtration
efficiency (FFE)
of various
consumer-grade
and improvised
face masks, as
well as several
popular
modifications of
medical
procedure masks.
Methods
Testing
Procedure
Fitted
filtration
efficiency tests
were conducted
between June and
August 2020 in a
custom-built
exposure chamber
(US
Environmental
Protection
Agency Human
Studies Facility
in Chapel Hill,
North Carolina)
as recently
described. The
institutional
review board at
the University
of North
Carolina at
Chapel Hill
waived the need
for study
approval as well
as individual
consent needed
for device
testing.
Briefly, a TSI
8026 Particle
Generator was
used to
supplement the
chamber with
sodium chloride
(NaCl) particles
that had a count
median diameter
of 0.05 μm
(range,
0.02-0.60 μm) as
measured by a
scanning
mobility
particle sizer.
The test
atmosphere was
allowed to
stabilize for 30
minutes before
FFE testing. The
chamber
temperature and
humidity during
testing ranged
from 73.4 °F to
85.1 °C and 10%
to 50%,
respectively.
The test
atmosphere used
for this study
reflects typical
indoor
conditions, with
exposure to
small particles
that are
slightly smaller
than individual
SARS-CoV-2
virions
(reported to
range between
0.06 μm and 0.14
μm). A sampling
port was
installed in
each mask using
a TSI model
8025-N95 Fit
Test Probe Kit
to allow
sampling behind
the mask. All
masks were
fitted on a man
(weight, 165.3
lb; height, 5 ft
and 10.1 in;
head size, 23.0
in) with no
beard. A pair of
TSI 3775
Condensation
Particle
Counters were
run in
single-particle
analysis mode to
continuously
monitor ambient
particles (0.02
μm-3 μm) in the
chamber just
outside the face
mask and
particles in the
breathing space
behind the face
mask at a
sampling rate of
1 second.
Fitted
filtration
efficiency
measurements
were collected
during a series
of repeated
movements of the
torso, head, and
facial muscles
as outlined by
the OSHA
Quantitative Fit
Testing Protocol
(Modified
Ambient Aerosol
CNC Quantitative
Fit Testing
Protocol For
Filtering
Facepiece Table
A–2—RESPIRATORS).
The FFE
corresponds to
the
concentration of
particles behind
the mask
expressed as a
percentage of
the particle
concentration in
the chamber air
and was measured
for the duration
of each test
described in the
OSHA protocol
(bending at the
waist, reading
aloud, looking
left and right,
and looking up
and down). The
overall
percentage of
FFE is
calculated as
100 ×
(1 − behind the
mask particle
concentration / ambient
particle
concentration),
and the
percentage of
FFE and the
standard
deviation were
calculated
across the
length of the
test. The total
testing time for
each mask was
approximately 3
minutes.
Products Tested
Two categories
of products were
tested for this
study:
consumer-grade
face masks and
medical
procedure masks
with and without
enhancements.
The following
consumer-grade
masks were
tested: (1) a
2-layer woven
nylon mask (54%
recycled nylon,
43% nylon, 3%
spandex) with
ear loops (Easy
Masks LLC)
tested with an
optional
aluminum nose
bridge and
nonwoven filter
insert in place,
(2) a cotton
bandana folded
diagonally once
“bandit” style
or in a
multilayer
rectangle
according to the
instructions
presented by the
US Surgeon
General
https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-to-make-cloth-face-covering.html),
(3) a
single-layer
woven
polyester/nylon
mask (80%
polyester, 17%
nylon, 3%
spandex) with
ties (Renfro
Corporation) (4)
a nonwoven
polypropylene
mask with fixed
ear loops (Red
Devil Inc), (5)
a single-layer
woven
gaiter/neck
cover balaclava
bandana (92%
polyester and 8%
spandex; MPUSA
LLC), and (6) a
3-layer woven
cotton mask
(100% cotton)
with ear loops (Hanesbrands
Inc).
The baseline FFE
of unmodified
medical
procedure masks
with elastic ear
loops (Cardinal
Health Inc) was
measured (n = 4)
and compared
with the FFE of
the same type of
mask with
various
modifications
designed to
enhance its
function. The
following
modifications
were tested: (1)
enhancing the
mask/face seal
by tying the ear
loops and
tucking in the
side pleats; https://youtu.be/UANi8Cc71A0),
(2) fastening
ear loops behind
the head with
3-dimensional–printed
ear guards; https://www.thingiverse.com/thing:4249113),
(3) fastening
ear loops behind
the head with a
23-mm claw-type
hair clip, (4)
enhancing the
mask/face seal
by placing a
ring of 3 ganged
rubber bands
over the mask,
with the center
rubber band
placed over the
nose and chin of
the participant
and the left and
right side bands
looped over each
ear;
“fix-the-mask”
3–rubber band
method https://www.youtube.com/watch?v=CVjGCPfRwUo),
and (5)
enhancing the
mask/face seal
by sliding a
10-inch segment
of nylon hosiery
over the fitted
mask.
Results
This study
evaluated the
FFE of 7
consumer-grade
masks and five
procedure mask
modifications.
The mean (SD)
FFE of
consumer-grade
face masks
tested in this
study ranged
from 79.0%
(4.3%) to 26.5%
(10.5%), with
the washed,
2-layer woven
nylon mask
having the
highest FFE and
the 3-layer
woven cotton
mask having the
lowest. The
cotton bandana
folded into a
multilayer
rectangle
affixed to the
ears with rubber
bands, as
described by the
US Surgeon
General,
provided a mean
(SD) FFE of
49.9% (5.8%).
Folding the
bandana bandit
style produced a
similar result
(mean [SD] FFE,
49.0% [6.2%]).
The tested mean
(SD) FFE of the
single-layer
woven polyester
gaiter/neck
cover balaclava
bandana was
37.8% (5.2%).
The single-layer
woven
polyester/nylon
mask, which is
attached with
tie strings,
tested at a mean
(SD) FFE of
39.3% (7.2%).
The nonwoven
polypropylene
mask with
nonelastic
(fixed) ear
loops tested at
a mean (SD) FFE
of 28.6%
(13.9%).
As expected
based on data
from our
previous work, a
National
Institute for
Occupational
Safety and
Health–approved
3M 9210 N95
respirator used
as a reference
control provided
very high mean
FFE (98.4%
[0.5%]; n = 1).
The medical
procedure masks
with elastic ear
loops tested in
this study had a
mean (SD) FFE of
38.5% (11.2%),
(which was lower
than that of
medical surgical
masks with tie
strings (71.5%
[5.5%]; n = 4).
Tying the ear
loops and
tucking in the
corners of the
procedure mask
to minimize gaps
in the sides of
the mask
increased the
mean (SD) FFE to
60.3% (11.1%).
The
“fix-the-mask”
3–rubber band
modification and
the nylon
hosiery sleeve
modifications,
which were also
intended to
reduce gaps
between the mask
and the wearer’s
face, improved
mean (SD) FFE to
78.2% (3.3%) and
80.2% (3.1%),
respectively.
Modifications to
improve the seal
of the mask
against the face
by increasing
the tension of
the ear loops
also improved
FFE. Attaching
the ear loops to
the ear guards
device using the
center hooks
(tightest
option)
increased
procedure mask
mean (SD) FFE to
61.7% (6.5%).
Similarly,
joining the ear
loops behind the
wearer’s head
using a
claw-style hair
clip increased
the procedure
mask mean (SD)
FFE to 64.8%
(5.1%). None of
the
modifications
tested enhanced
procedure mask
FFE to the level
of an N95
respirator.
Discussion
In this study,
consumer-grade
masks and
medical
procedure mask
modifications
were tested as
personal
protective
equipment
(protection for
the wearer)
against a test
aerosol of
0.05-μm NaCl
particles.
Although the FFE
of
consumer-grade
masks and face
coverings was
variable, the
FFE of some
consumer-grade
products
exceeded that of
medical-grade
procedure masks.
For example, the
2-layer woven
nylon mask with
ear loops was
tested under
various
conditions,
including with
and without an
aluminum nose
bridge, with and
without a
commercially
available
nonwoven insert,
and after 1 wash
cycle in a
standard
household
washing machine
(air-dried on a
drying rack).
The unwashed
nylon mask
without a nose
bridge or insert
had an FFE of
44.7%. The
addition of a
nose bridge
reduced visible
gaps around the
nose and
increased FFE to
56.3%. Adding a
nonwoven filter
insert to the
mask with the
nose bridge in
place resulted
in a further
increase in FFE
to 74.4%.
Interestingly,
the FFE of the
nylon mask (with
the nose bridge
but without the
filter insert)
improved
slightly to
79.0% after
washing. It is
unclear why
washing alone
improved the FFE
from 56.3% to
79.0%. It may be
that the
washing/drying
process
unraveled some
of the fibers to
increase the
overall
filtration
surface, and
thus filtration
efficiency, of
the medium, or
perhaps it
modified the
mask shape or
size in a way
that improved
fit, or both.
The
washing/drying
test was not
repeated with
additional nylon
masks. Further
investigation to
assess the
association of
single and
multiple washing
with mask
integrity and
material
disposition
would be
necessary to
validate any
improvement in
FFE.
The woven cotton
mask, which
comprises 3
layers and has a
thin, flexible
metal nose
bridge, had the
lowest FFE in
this study
(26.5%). The
relatively loose
weave of the
cotton layers,
while providing
improved
breathability
and comfort, may
reduce
filtration
efficiency.
Additionally, we
evaluated the
FFE of
improvised face
coverings,
including a
standard cotton
bandana and a
neck gaiter
balaclava
bandana. The
cotton bandana,
when folded
either bandit
style or
according to the
US Surgeon
General’s
instructions,
achieved
approximately
50% FFE, which
is better than
the ear loop
procedure mask
we tested. Neck
gaiter balaclava
bandanas have
also emerged as
a popular face
covering,
particularly
among athletes
and young
adults. As
tested in this
study, the
single-layer
gaiter, which
was made of 92%
polyester and 8%
spandex and fits
tightly to the
wearer’s nose
and mouth, had
an FFE of 37.8%.
While this face
covering
appeared to fit
the wearer well,
with no visible
gaps in the
seal, it may be
that the
relatively low
FFE can be
attributed to
the low
filtering
efficiency of a
single thin
layer of woven
material with
large porosity.
For medical
procedure masks,
modifications
that enhanced
the fit between
the mask and the
wearer’s face
improved FFE.
Simply tying the
ear loops and
tucking the
corners of the
mask against the
wearer’s cheeks
visibly improved
mask fit and
increased FFE
from 38.5% to
60.3%. The most
effective
modification
tested was the
use of a nylon
hosiery sleeve
placed over the
procedure mask.
This
modification,
which held the
mask tight to
the wearer’s
face, eliminated
all visible gaps
and increased
FFE from 38.5%
to 80.2%.
However, donning
the nylon sleeve
over the
procedure mask
was cumbersome
and limited the
wearer’s ability
to adjust the
procedure mask.
Generally,
improvements in
procedure mask
FFE appeared to
be associated
with the
integrity of the
seal of the
edges of the
mask to the
wearer’s face,
demonstrating
the importance
of mask fit to
maximizing
filtration.
While all of the
modifications
described
enhanced
protection
against airborne
particles for
the wearer, not
all were
comfortable or
practical for
extended use.
For example, the
3–rubber band
“fix-the-mask”
modification
created
considerable
pressure on the
wearer’s ears,
making it
uncomfortable
after only
minutes of wear
and raising
questions about
its adoption by
the general
public. While
the
modifications
shown in this
article can
improve mask fit
and provide
increased
filtration of
airborne
particles, it is
important to
choose a
modification in
which discomfort
is not a
deterrent from
wearing the mask
for prolonged
periods.
The full text is
included in the
link below to an
article in the
Journal of
the American
Medical
Association.
N95 Tested at
98% FFE
3M™
says its
Aura™ Series
Particulate
Respirator
9210+, N95 is a
breakthrough in
comfort and
convenience.
This
three-panel,
flat-fold
disposable
respirator with
its innovative
design helps
provide
comfortable,
reliable worker
protection
against non-oil
based particles.
The lightweight,
three-panel
designed
disposable N95
particulate
respirator helps
provide quality,
reliable, and
convenient
worker
respiratory
protection. 3M
uses a variety
of innovative
technologies and
features to help
meet respiratory
protection and
comfort needs.
3M`s proprietary
filter media,
3M™ Advanced
Electret Media,
filters dust and
other particles,
while allowing
for easy
breathing. The
soft inner
material
provides added
comfort while
the soft nose
foam and
adjustable nose
clip help
provide a custom
seal. Braided
headbands
provide comfort
and help
minimize pulling
of hair.
Unique features
to the Aura™
Series
Particulate
Respirators
include sculpted
nose panel that
follows the
contours of the
nose allowing
more room for
eyewear,
embossed top
panel that is
designed to help
reduce the
fogging of
eyewear from
warm, moist
exhaled air, and
innovative chin
tab designed for
ease of
positioning,
donning, and
adjustment.
These features
are designed to
enhance user
comfort and help
increase
wearability. The
unique
three-panel flat
fold design is
collapse
resistant and
its individual
packaging allows
for easy storage
prior to use.
Suggested
applications:
Grinding,
Sanding,
Sweeping,
Bagging and
other dusty or
arid operations.
Can also be used
to help reduce
inhalation of
certain airborne
biological
particles like
mold, Bacillus
anthracis,
Mycobacterium
tuberculosis,
etc. Example
applications
include
emergency or
pandemic
preparedness
planning,
stockpiling,
etc.
