
Coronavirus
Technology Solutions
November 18, 2020
COVID is a Lottery
Where You Want to Minimize the Number of Tickets
Lonza to Make Moderna
Vaccine in the U.S. and Switzerland
EU Approves 4th
Contract with Pfizer
Sonovia Mask Relies on Anti-Microbials Rather
than Sub-Micron Filtration
U.S. Death Toll Passes 250,000
_________________________________________________________________________
COVID is a Lottery
Where You Want to Minimize the Number of Tickets
Covid is a lottery
you want to lose. If you live in the U.S. you
presently hold about 3% of the winning tickets
to win a prize and about 0.5% of the tickets for
the grand prize (death). These are not good
odds. As a driver or passenger in a country with
a 65 mph speed limit you hold 0.5% of the
tickets to win the grand prize. But this is over
an entire life time and not just six months.
We are willing to
take the risks associated with automobiles
because of the benefits. If we cut the speed
limit back to 55 mph we would increase life
expectancy by only a few months. So this is an
acceptable risk. Our COVID strategy should be to
reduce the number of winning tickets until they
are comparable to automobile travel.
Relative
to masks the percent of winning tickets depends
on
·
virus load
·
mask performance
·
mask use (in part a
function of comfort and attractiveness)
McIlvaine has
compared the combination of fit and efficiency
to determine mask performance of CATE masks
(Comfortable, Attractive, Tight fitting,
Efficient) to cloth masks, surgical masks and
loose fitting
N95 masks. CATE masks reduce the number
of lottery tickets by seven fold vs the others
if worn for identical times. If due to comfort
and attractiveness CATE masks are used
more frequently this further reduces the
number of tickets.
CATE masks can be
supplied with various levels of efficiency.
Normal leakage is 2% to 8%.
Media efficiency can be 90%, 95% or 99%.
So with the highest efficiency and highest
leakage you are equivalent to the lowest
efficiency and lowest leakage.
Mask use is the other
variable. When the virus load is high e.g.
equivalent to the risk of traveling routinely at
100 mph everyone will want to wear a mask. But
in a situation where the viral load risk is
equivalent to traveling at 65 mph many people
will discard their masks. If they are wearing
comfortable and attractive masks they will be
more inclined to wear masks at this risk level.
Mask selection needs to be made on this holistic
basis with load, performance and use all
considered. It is likely that most people will
want multiple mask designs which are appropriate
for different levels of risk.
The goal will be to match the mask
selection with a risk level equivalent to the 65
mph speed limit.
Lonza to Make Moderna Vaccine in the U.S. and
Switzerland
Swiss drug maker Lonza has
partnered with Moderna and says it aims to
produce 400 million doses of the vaccine
annually. The U.S. firm is aiming for 500
million to 1 billion doses in total for 2021.
Anyone receiving the vaccine will require two
doses, as with Pfizer’s shot, showing how long
it could take, with the current manufacturing
capacity, to vaccinate internationally.
Lonza will produce ingredients within Moderna’s
vaccine, formally called mRNA-1273, in
facilities in the U.S. and Switzerland, where it
is headquartered. Company Chairman Albert Baehny
told CNBC about the “big challenges” facing drug
makers like his when it comes to scaling up
production.
“We can only produce more than 500 million doses
a year if we install additional manufacturing
lines, so it is clear that we need additional
investments in installation if we want to
produce more than 500 million (per year) in the
future,” he told CNBC’s “Squawk Box Europe” on
Wednesday.
Baehny identified more challenges to vaccine
production that the company had had to confront
since embarking on its partnership with Moderna.
“There are a few issues, the first is speed. We
only started 10, 11 months ago and now we are
producing the first commercial batches of the
drug substance in North America, and we are
planning the first batch of commercial volume in
one or two weeks in Switzerland, so the speed
has been a challenge.”
“The second challenge is to find the people. For
each manufacturing line you need 60-70 educated
persons. We’ve installed four manufacturing
lines so you have to identify and train these
people,” he said.
“Then linked to the speed (issue), you have to
have access to the equipment, install the
equipment, and then test your manufacturing
facility, so (these are) big challenges,
resolved, or almost resolved, in less than one
year.”
Temperature, and keeping the vaccines cold
enough during transportation, is another big
challenge.
Pfizer’s vaccine requires a storage temperature
of minus 94 degrees Fahrenheit, or -70 degrees
Celsius. By comparison, Moderna said on Monday
that its vaccine remains stable at 36 to 46
degrees Fahrenheit — the temperature of a
standard home or medical refrigerator — for up
to 30 days. It can be stored for up to six
months at minus 4 degrees Fahrenheit.
“Those are standard conditions in the
pharmaceutical industry,” Baehny said. “So I
don’t see many problems for the distribution,
for the shipping and for the storage of
Moderna’s vaccine,” he said.
Lonza Biologics in Portsmouth NH has ramped up
its production of the vaccine in anticipation of
final approval for worldwide distribution.

"The Lonza Portsmouth site commenced production
in July and began large-scale production at the
end of September,” Lonza spokesman Glenn Myers
said Monday.
“We can't say how much vaccine has been
produced; however, our goal is to produce drug
substance from our Portsmouth manufacturing
suites for 100 million doses per year,” he
added.
Lonza is also bulking up its workforce. “We have
scaled up our production workforce with 70
dedicated employees with a plan to scale to 100
employees as quickly as possible," said Myers.
Lonza CEO Pierre-Alain Ruffieux offered his
congratulations on Moderna’s phase 3 clinical
trial data of its vaccine, called mRNA-1273.We
are proud to support Moderna in the production
of mRNA-1273. Our collaboration will allow for
the manufacture of material equivalent to 400
million doses per year from Lonza’s facilities.
Large-scale production began at our Portsmouth,
U.S., site at the end of September and we are on
track to begin production at our Visp,
Switzerland, site before the end of the year,”
Ruffieux said.
“With positive interim results from both
Moderna’s and Pfizer’s mRNA vaccine candidates,
we are witnessing a major step forward in the
evolution of vaccine technology, with the
potential to change the way we manage infection
and disease in the future,” he added.
EU Approves 4th Contract with Pfizer
The European Commission approved a fourth
contract with pharmaceutical companies BioNTech
and Pfizer, which provides for the initial
purchase of 200 million doses on behalf of all
EU Member States, plus an option to request up
to a further 100 million doses, to be supplied
once a vaccine has proven to be safe and
effective against COVID-19. Member States can
decide to donate the vaccine to lower and
middle-income countries or to re-direct it to
other European countries.
Today's contract with the BioNTech-Pfizer
alliance builds upon the broad portfolio of
vaccines to be produced in Europe, including the
already signed a contracts with AstraZeneca, Sanofi-GSK and Janssen
Pharmaceutica NV, and
the concluded successful exploratory talks with CureVac and Moderna.
This diversified vaccines portfolio will ensure
Europe is well prepared for vaccination, once
the vaccines have been proven to be safe and
effective.
President of the European Commission, Ursula von
der Leyen, said: “In the wake of
Monday's promising announcement by BioNTech and
Pfizer on the prospects for their vaccine, I'm
very happy to announce today's agreement with
the European company BioNTech and Pfizer to
purchase 300 million doses of the vaccine. With
this fourth contract we are now consolidating an
extremely solid vaccine candidate portfolio,
most of them in advanced trials phase. Once
authorized, they will be quickly deployed and
bring us closer to a sustainable solution of the
pandemic.”
Stella Kyriakides,
Commissioner for Health and Food Safety, said: “A
safe and effective vaccine is the only lasting
exit strategy from the pandemic, and is at the
centre of our European Vaccine Strategy. Today's
agreement follows the encouraging first
indications from the clinical trial results and
is further evidence of our commitment to putting
more Europe in the area of health. It is a very
telling example of what the EU can achieve when
working together, as a Union, and a case in
point of what a future European Health Union
will be able to deliver.”
BioNTech is a German company working with
US-based Pfizer to develop a new vaccine based
on messenger RNA (mRNA). mRNA plays a
fundamental role in biology, transferring
instructions from DNA to cells' protein making
machinery. In an mRNA vaccine, these
instructions make harmless fragments of the
virus which the human body uses to build an
immune response to prevent or fight disease.
The Commission has taken a decision to support
this vaccine based on a sound scientific
assessment, the technology used, the companies'
experience in vaccine development and their
production capacity to supply the whole of the
EU.
Vaccine doses for Europe will be produced in
BioNTech’s German manufacturing sites, as well
as in Pfizer’s manufacturing site in Belgium. If
the BNT162b2 vaccine candidate receives approval
from the European Medicines Agency (EMA),
then doses will be ordered by the EU Member
States who have elected to receive the vaccine
as part of this agreement.
Pfizer’s manufacturing plant in Portage played
a big role in the development of the vaccine but
so far production is being limited to the
Belgian site. Pfizer is headquartered in
Manhattan. The company went through several
hands going back to its roots as the Upjohn
Company in Kalamazoo. The company reached a
massive $2 billion deal with the U.S. government
earlier this year, along with its joint venture
partner in the vaccine, BioNTech. Pfizer is
planning to produce as many as 100 million doses
of the vaccine this year. Next year – that
skyrockets to 1.3 billion doses. It is a
two-dose vaccine. That makes production,
distribution, and keeping track of jabs all the
more important.
Sonovia Mask Relies on Anti-Microbials Rather
than Sub-Micron Filtration
The SonoMask is made of a fabric impregnation of
zinc oxide nano particles, which has proven to
have a strong anti-viral effect in laboratory
testing. The fabric has also been tested to
successfully last throughout 55 wash-cycles,
with a filtration rate of 5μm, in accordance
with WHO guidelines. Tests by Taryag
Laboratories showed more than 98% efficiency on
5 micron particles.
It should be noted that N95 masks are
tested using 0.1 micron particles with
efficiency rating being based on 0.3 micron
particles. So the efficiency test method is not
comparable. It claims high overall protection
but this must be based on anti-microbial
features rather than particle removal.
The Wall St. Journal
wrote in May “Sonovia had been working toward
selling machines and chemicals used to add
antibacterial and antiviral properties to
fabric, and it began manufacturing masks using
the technology two months ago, said Liat
Goldhammer, the company’s chief technology
officer. The fabric used in the masks has been
tested successfully on other coronaviruses, and
Sonovia is awaiting the results of recent tests
on the novel coronavirus. They have sold
thousands of masks to consumers in the U.S. and
have also donated thousands to hospitals in
South Africa, Israel and Germany.
The sono-chemical coating methodology was
developed in 2013 at the
Bar Ilan University in Israel. Sonovia’s
team, which includes Chemistry
Nobel Prize Winner Prof. Sidney Altman,
and has used the company’s proprietary formula
to significantly decrease the transmission and
impact of hospital acquired infections globally.
The question is what impact the anti-viral nano
particles have on small virus particles which
pass through the mask. It would seem that there
would be no contact or if there were contact it
would be so brief as to not be meaningful. On
the other hand the material could be effective
on viruses captured by the mask. This could
reduce surface transmission from the mask.
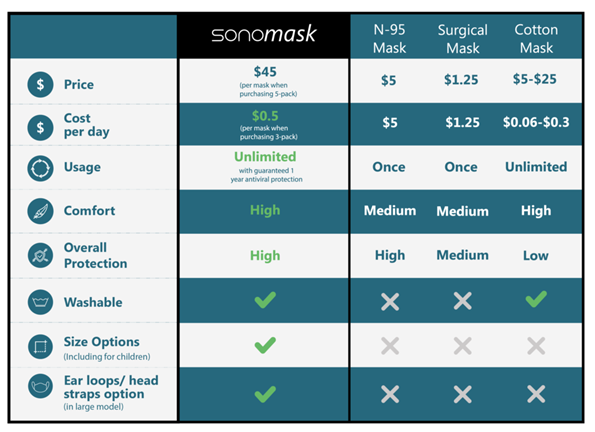
Here is the comparison with other masks prepared
by Sonovia.
Besides the efficiency concern another area of
investigation is health impacts related to
nanoparticles.
One researcher believes that this is an
area where more study is needed.
U.S. Death Toll Passes 250,000
The United States passed a grim milestone on
Wednesday, hitting 250,000 coronavirus-related
deaths, with the number expected to keep
climbing steeply as infections surge nationwide.
Experts predict that the country could soon be
reporting 2,000 deaths a day or more, matching
or exceeding the spring peak, and that 100,000
to 200,000 more Americans could die in the
coming months.
Just how bad it gets will depend on a variety of
factors, including how well preventive measures
are followed and when a vaccine is introduced.
“It all depends on what we do and how we address
this outbreak,” said Jeffrey Shaman, a Columbia
University professor of environmental health
sciences who has modeled the spread of the
disease. “That is going to determine how much it
runs through us.”
Back in March, when the virus was still
relatively new and limited mainly to a few
significant pockets like New York, Dr. Anthony
S. Fauci, the top infectious disease expert in
the country, predicted that it might kill up to
240,000 Americans.
It has now passed that mark, with no end in
sight.
Since the very beginning, preventive measures
like wearing masks have been caught up in a
political divide, and that remains the case
today, as the Trump administration resists
beginning a transition of power to
President-elect Joseph R. Biden Jr. and
cooperating on a pandemic strategy.
New vaccines may begin to have an impact next
year, experts said, and for now, developments in
treating the disease as well as a younger
population getting infected mean that far fewer
people who are admitted to hospitals are dying.
Infections are also being diagnosed
earlier, which helps combat it.
The deadliest day of the pandemic in the United
States was April 15, when the reported daily
toll hit 2,752.
There is always a lag in deaths, compared with
the rate of infection and hospitalizations, and
with the latter measure now hitting records
every day — 76,830 Americans were hospitalized
on Tuesday, according to the Covid
Tracking Project — the death toll is certain to
go on rising.
Covid-19 deaths have continued their bleak march
with little respite throughout the year.
By March 24, a little over a month into the
pandemic, 50,000 people had died. That number
doubled to 100,000 by May 27 and added another
50,000 within two months, by July 29. Two months
later, on Sept. 22, the total reached 200,000.
Toward the end of the summer, the number of
cases being reported daily in the United States
eased, after a brief spike in July. But they
have been soaring again since the beginning of
November.
On Sept. 22, there had been somewhat more than
6.9 million total cases in the United States,
according to
a New York Times database.
As of today, there have been more than 11.5
million.
The combination of the onset of winter, fatigue
with preventive measures, holiday travel and
gatherings as well as the patchwork of responses
across all 50 states is expected to drive that
number still higher.
“We have a lot of people who have not been
infected with this yet,” said Dr. Shaman. “If
you get complacent, the virus does not care. It
is just going to come back.”
