
Coronavirus
Technology Solutions
October 22, 2020
Efficient, Tight Fitting Masks are Affordable
Comfort can be Combined with Efficiency and a
Tight Fit
Table Top UV Air Sanitizer Being Used in Schools
PureAir and Noble Biomaterials Partner on
Antimicrobial Filter
Vietnamese Company has Mask Which Captures and
Destroys COVID
Stocks Which will Benefit from the COVID Battle
______________________________________________________________________________
Efficient, Tight Fitting Masks are Affordable
Efficient tight fitting(ETF)
masks are
capable of removing
95% of the virus whereas loose fitting
cloth masks will remove only 20%.
It is
therefore important that three billion people
wear these higher performing masks. The problem
is that their initial cost could be 50 or 100
times that of a surgical mask. However, since
many ETF masks do not rely on electrostatic
charging the efficiency is not negatively
impacted by washing. This means that
ETF masks can be cost effective.
Many ETF masks can be worn for months or even a
year depending on
·
number of hours per day of use
·
environmental conditions including air
pollutants
·
viral load
·
economic status
·
unusual damage
·
appearance
The general public will wear masks only a few
hours per day. The masks will be subjected to
air pollutants. The average viral load when even
in a crowded subway would be 0.01% of that of a
surgical nurse in a COVID ward. In the grocery
store with social distancing the potential viral
load could be 0.001%. This is important in
determining how often some sort of cleaning
might be necessary. A surgical nurse in a COVID
ward needs a mask discarded or cleaned for every
hour of use.
A person just traveling to the grocery
store could use the mask for 100,000 hours
before the same viral load will be collected. If
the person uses a mask only 500 hours per year
they only need to clean it once every 200 years.
Another aspect is that viruses do not survive
for 200 years.
They may only survive for 200 hours. The
takeaway is that discarding or excessively
cleaning EFF masks is not very beneficial as a
COVID protection.
The newest breed of ETF masks do not rely on
electrostatic charging and can be cleaned
repeatedly. If a $30 mask is used 100
times it is less expensive than most single use
masks and much more effective (93% vs 20%).
ETF masks will eventually capture sufficient air
pollutants that the resistance will increase and
make breathing more uncomfortable. Over time
they will not have a clean appearance despite
hand washing or alcoholic sprays. This means
that anyone above the poverty line could dispose
of a mask while it is still efficient at
removing virus.
At one extreme you will have decisions by the
wealthy which do not consider the cost of a $30
mask to be significant. The average individual
can easily afford to replace masks every few
months.
For those below the poverty line an
expenditure will need to be limited to a few
dollars per year or governments will want to
provide a subsidy.
The cost for a specific individual is a function
of each of the factors. The following graph
shows three scenarios where all the variables
are low, medium or high.
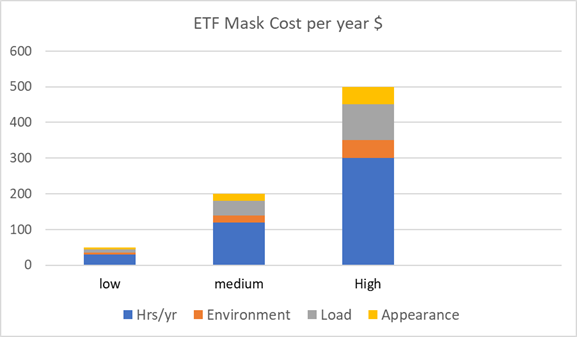
The result is a yearly cost of $50 to $500
per
person. If 3 billion people wear masks with half
spending only $50/year each the cost would be
$675 billion.
|
# in millions |
Cost/yr/person $ |
Cost $ millions |
|
500 |
500 |
250,000 |
|
1000 |
200 |
200,000 |
|
1500 |
50 |
225,000 |
|
Total |
|
675,000 |
Considering that the Cost of COVID is 50 times
higher this is a very worthwhile investment.
Comfort can be Combined with Efficiency and a
Tight Fit
Vogmask has succeeded in combining comfort along
with efficiency and a tight fit with their
latest mask design. Inhalation resistance is a
small fraction of the 30 ml maximum
for workplace respirators as established by
NIOSH. Here are test results just compiled by
Nelson Labs.
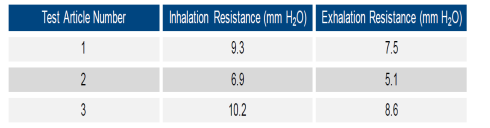

Table Top UV Air Sanitizer Being Used in Schools
Boski Corporation, manufacturer of a new
airborne sanitation device, today announced the
deployment of their flagship product, the
tabletop UV-C Rapid Air Sanitizer, throughout
schools and businesses across the US. With the
COVID-19 pandemic raging on and influenza season
officially starting this fall, the availability
of Boski's product couldn't come at a more
appropriate time for schools such as Susquehanna
University and the University of Colorado
Denver.
Unlike other methods of UV-C light
sanitation which can be harmful to human eyes
and skin, and require a totally vacant space,
the Boski UV-C Rapid Air Sanitizer is safe for
people, as the UV-C light sanitation process is
contained within the enclosed device.

Because the device can provide added layers of
protection against airborne virus transmission
at an affordable cost, numerous organizations
have already adopted Boski's technology, some on
a larger scale. Susquehanna University is a
prime example, having placed nearly 1,000 Boski
devices across their Selinsgrove, PA campus as
part of their pandemic risk mitigation strategy.
"UV-C light is a proven technology we are
already using for various applications across
campus," explains Chris Bailey, Susquehanna's
Director of Facilities Management. "For air
treatment, we weren't going to be able to
quickly implement UV-C into all of our large air
handlers due to expense and availability. Boski
delivered a great price point, the device is
very simple to use and it's extremely safe,
which made it a perfect supplemental fit to our
overall program. We've currently placed over 900
units in student dorm rooms and by the time
we're done, we'll have deployed almost 1,000
units across campus in residence halls, music
rooms and office space. It sends a great message
to parents as well – we've received positive
feedback and appreciation from parents about the
extra measures Susquehanna is taking to keep
students safe."
"We purchased Boski UV-C units as a way to
increase safety for our students and staff that
were in on-campus labs," said James Salmen, M.S.
Lab Manager/Director of Facilities Department of
Integrative Biology at the University of
Colorado Denver. "It was one more thing we could
do to make student and staff health our top
priority."
Designed by Dr. Hooman Banaei, Boski's
co-founder and CTO with a PhD in Electrical
Engineering, Boski differs from similar
products. Unlike other methods of UV-C light
sanitation which can be harmful to human eyes
and skin, and require a totally vacant space,
the Boski UV-C Rapid Air Sanitizer is safe for
people, as the UV-C light sanitation process is
contained within the enclosed device. Typical
air purifiers focus on removing physical
pollutants, like dust dander, ash and other
particles from the air, with less of a focus on
killing viruses. Boski's exclusive focus is
attacking airborne viruses, using the optimal
blend of high efficacy UV-C light technology
with light/air residence time. The UV-C
light within Boski's device is rated at 254
nanometers, which falls within the most lethal
range of UV-C to viruses. Studies on UV-C light
have shown that UV-C at 254 nanometers can
inactivate SARS-CoV-2 as well as harder to kill
pathogens.
Many similar devices have not undergone third
party lab testing for efficacy against killing
airborne viruses. In October of 2020, Boski
completed third party lab testing with ARE Labs
in compliance with the FDA Good Laboratory
Practices (GLP) as defined in 21 CFR, Part 58.
Results showed a kill rate of 99.99%+ of MS2, a
non-enveloped virus which are typically harder
to kill than enveloped viruses like COVID-19.
PureAir and Noble Biomaterials Partner on
Antimicrobial Filter
PureAir Filtration has partnered
with Noble Biomaterials to launch
FiberShield™, the first antimicrobial product
with silver technology that can be used in any
filter.
FiberShield™ is made of a proprietary blend of
nonwoven nanofibers that are impregnated with
antimicrobial Ionic+™ silver technology. The
antimicrobial fabric can be used in any
particulate filter and is the only one on the
market to offer such flexibility to filter
manufacturers. FiberShield™ with Ionic+
technology has been tested and proven effective
by independent testing laboratories to
inactivate over 99% of specific pathogens.
PureAir also debuted a second product in its
antimicrobial line called Microbe-sorb™, an
adsorbent media that utilizes a proprietary
blend of compounds to activate, enhance and
deliver the strong antimicrobial properties of
permanganate, a material commonly used in
medical practices since the early 1800s.
Independent laboratory tests show Microbe-sorb
inactivates over 99% of microbes on contact.
Both of PureAir’s new products are aimed at
mitigating the impact of the COVID-19 pandemic
by focusing on improving air quality through
gas, odor and pathogen removal.
PureAir Filtration’s systems are custom designed
and focus on four key areas: Odor & Emission
Control, Protecting Electronics, Indoor Air
Quality, and Toxic Gas Protection. PureAir
provides a line of products that simplify the
regular maintenance of its systems. This
includes a line of real-time electronic air
quality monitoring, real-time media bed life
monitoring, chemical filtration, corrosion
classification coupons and media life analysis.
Noble Biomaterials, Inc. is a global leader in
antimicrobial and conductivity solutions for
soft-surface applications. The company produces
advanced material technologies designed for
mission-critical applications in the performance
apparel, healthcare, industrial and emerging
wearable technology markets. Its flagship
brands, X-STATIC®, Ionic+™ and CIRCUITEX®, are
used by hundreds of licensees to provide odor
elimination, infection prevention/management,
biometric monitoring and conductive protection
benefits.
Vietnamese Company has Mask Which Captures and
Destroys COVID
Wakamono is a Nano Biotechnology company that
was established in 2010 in Vietnam. They have
been successful in the production of the world’s
first anti coronavirus surgical mask. This
surgical face mask has shown its effectiveness
against 99% of SAR-CoV-2 strains.
Wakamono surgical masks are also effective
against influenza A H1N1 (enveloped viruses),
poliovirus-I (non-enveloped viruses), and 99% of
harmful bacterial pathogens.
The mask’s efficiency was tested and certified
by reputable and reliable independent
laboratories following the standard ISO
18184:2019. Additionally, Wakamono surgical
masks could successfully meet and exceed the
US's highest FDA standards, i.e., ASTM F2100
level 3 and European CE EN 14683 Type IIR.
An N95 medical respirator can filter out 95% of
particles
approximately 0.3-micron size, as stated above.
However, the novel coronavirus is approximately
0.05 – 0.2 micron. It is always advised that
users should not touch the mask surfaces to
avoid contamination on either side. The filtered
pathogenic microorganisms adhered to or trapped
on the mask's surface do not die but continue
their life cycle. For example, coronavirus lives
for up to seven days on disposable masks.
While addressing these problems, the scientists
at Wakamono developed the surgical mask with
dual properties, i.e., filtration of harmful
pathogens and destruction of trapped viruses and
bacteria.
In the production of the multilayered surgical
mask, Wakamono has utilized Gecide fabric. These
fabrics provide antibacterial property against
harmful bacterial pathogens. The inner layer of
the mask comprises Gecide fabric layer. This
inner layer is covered with millions of organic
nanoparticles. These nanoparticles can form
traps to weaken and destroy harmful pathogens
(virus and bacteria). All the biocide organic
and natural components used in the development
of the surgical masks are safe in accordance
with the in vitro cytotoxicity ANSI/AAMI/ISO
10993-5:2009 by Pacific Labs (USA). Wakamono
masks do not utilize any metal nanoparticles in
the masks.
The use of Wakamono surgical masks could
significantly decrease the infection rate as it
would effectively reduce virus transmission.
Therefore, this development can act as a
potential tool to combat the COVID-19 pandemic.
Stocks Which will Benefit from the COVID Battle
Three stocks which could benefit from the fight
against COVID were reviewed by
Edward Vranic, CFA
of Long/Short Equity,
Honeywell International Inc. (HON)
recognized the COVID opportunity months
ago, launching its Healthy Buildings solutions
in May. The company is able to combine its air
quality, safety and security products with
advanced analytics in order to provide building
managers with personalized service to monitor
for and minimize any potential virus outbreak.
The stock has underperformed the market since
the virus outbreak despite the launch of this
solution and its heavy involvement in
distributing PPE. So investors who are looking
for an under-the-radar stock that has benefited
from the coronavirus economy might have one in
HON.
Honeywell's opportunity is enormous and varied,
as evidenced by a recently
announced collaboration with the Carolina
Panthers NFL team to manage the safe return of
fans back to the team's home stadium. The
Healthy Buildings solutions will continuously
monitor the air quality within Bank Of America
Stadium and Honeywell will provide fans with
Panthers-branded hygiene and protective gear
such as masks, hand gel and cleaning wipes.
Given the resistance of some people in the
United States to obeying safety protocols which
has led to further spread of the virus, the
branded gear might help to encourage proper use.
Once the threat of COVID-19 subsides, the
Honeywell solution can morph into an IoT
building management service. In addition to
monitoring for the virus, it will identify and
correct building controls issues such as carbon
dioxide levels, temperature and humidity. The
Healthy Buildings dashboard can manage aspects
of building management like fan capacity and
cleaning tracking. This can lead to cost savings
and service improvements as resources and
employees such as janitors can be more timely
and efficiently deployed. The virus acts like a
door-opener for Honeywell's building management
solutions that could lead to permanent new
business that might otherwise have been a harder
sell without the immediate urgency.
Kontrol Energy Corp. (OTCQB:KNRLF)
(KNR.C) recently created what it calls BioCloud
technology in order to manage the pandemic. This
wall-mounted unit detects the presence of
COVID-19 in the air and triggers an alert system
to warn building managers so moves can be made
to control an outbreak before it occurs.

Last month, the company was featured in an
article by the CBC (Canada's government-owned
national news media outlet), causing the stock
to more than double in price after it already
had a good run. It currently has a market cap of
approximately $110 million. The immediate target
market will be schools, hospitals, long-term
care homes and mass transit. The company
estimates a $12,000 price tag for each unit and
says it has secured the manufacturing capacity
to produce up to 20,000 units per month. The
technology was independently tested so the next
step would be securing contracts.
In addition to BioCloud, the company recently
procured a new order for its SmartSuite® smart
building technology across four apartment
buildings located in Ontario, Canada. So much
like how Honeywell is able to leverage its virus
management solutions into an IoT building
management contract, Kontrol may be able to do
the same thing on a smaller scale.
While Kontrol has developed a virus tracker and
early warning system, Manganese X Energy Corp. (OTCPK:MNXXF)
(MN.V) is looking to go one step further and
outright kill the virus once detected in a
building. Earlier this month, its subsidiary
Disruptive Battery Corp. signed an MOU with
PureBiotics to acquire a significant equity
share of up to 50% in a PureBiotics
Environmental Air Quality Control Company. Even
though it's still in the MOU phase, PureBiotics'
CEO Lino Morris recently joined the company's
Advisory Board.
The two companies are combining forces to
develop a product that will circulate air
disinfection agents through a building's HVAC
distribution system. It leverages Manganese X's
patented disinfection apparatus system with
PureBiotics' existing product lines and
technologies in order to cleanse buildings of
COVID-19 and any other pathogens. Lino Morris
has over 40 years’ experience in the
pharmaceutical industry. That experience will
come in handy as he will be the one spearheading
additional testing and validation of the
product. He is reaching out to universities to
test the air quality control delivery system.
Just like Kontrol, Manganese X wants that third
party validation of its product.
Manganese X sits at around a $25 million market
cap, a fraction of Kontrol's valuation as the
additional hurdle of validation needs to take
place. If validation is successful, Kontrol
represents a demonstrated upside for Manganese X
shareholders. The stock has already moved up
healthily over summer prices and has experienced
volatility recently. Hype over Tesla's (TSLA)
recent inclusion of Manganese in its battery and
desire to source battery materials is the
primary reason for the increase. Manganese X
owns the Battery Hill Manganese project situated
just north of the U.S. border in New Brunswick.
I briefly outlined it and the company's familial
connection to Tesla in a previous article.
Manganese X shareholders have a reduced risk
from diversification built in due to these two
disparate lines of business. Should one not pan
out, that has absolutely no impact on the
probability of success for the other.
Without getting too heavily into speculation,
assuming that both solutions from Kontrol and
Manganese X progress as hoped, it would make a
lot of sense for a building manager to have both
products on hand. BioCloud to detect COVID-19
and the Manganese X solution to cleanse the
virus through the HVAC system.
While Honeywell offers a virus building
management play for conservative investors,
these type of small cap companies are more
attractive to risk-tolerant small cap investors
such as myself. Both Kontrol and Manganese X
have moved up a lot in the past few months, so
speculative investors are no longer "buying low"
and have to be prepared for volatility. Early
stage investors who are in at lower prices might
choose to take their profits along the way. But
based on Kontrol's market cap of approximately
$110 million and Manganese X's market cap of
approximately $25 million, they leave a lot of
room for upside. I will continue to monitor the
small cap sector for indoor virus management
opportunities such as Kontrol Energy and
Manganese X.
