
Coronavirus
Technology Solutions
October 15, 2020
Experts say Masks are More Important Now than
Vaccines but Fail to Qualify the Term to Mean
“Efficient and Tight Fitting”
FDA will No Longer Review Chinese Masks for
Addition to the Approved List
Qualitative vs Quantitative Fit Tests
Polymer Face Masks Developed at Ag School and
Being Tested at Meat-Processing Plants
How Efficient are Masks Once Leakage is
Included?
OSHA will Levy Fines for Failure to Have
Appropriate Mask Fit Testing
Vogmask is a Lightweight Mask with a Good Fit
______________________________________________________________________________
Experts say Masks are More Important Now than
Vaccines but Fail to Qualify the Term to Mean
“Efficient and Tight Fitting”
McIlvaine agrees with the recent comments that
masks are the most important weapon in the fight
against COVID.
But using the term “mask” is
like using the term “pill”.
If you have malaria the quinine pill is a
solution but aspirin is not. A squirt gun is
nominally a gun but not the weapon of choice
against a deadly enemy.
To fight COVID the tight fitting efficient mask
is the solution. A loose fitting cloth mask is
no better a solution than an aspirin.
Some are expressing concern that people
over estimate the protection given by poorly
fitting inefficient masks. Here are recent
comments by those who do not define the term
“mask”
While there has been a lot of talk and hope that
an effective Covid-19 vaccine will be available
soon, top experts say an affordable tool that
already exists is just as important in the fight
against the pandemic: masks.
Dr. Christina Brennan, vice president of
clinical research at Northwell Health’s
Feinstein Institutes for Medical Research,
told “60 Minutes” on Sunday that if she had to
choose between a mask or a vaccine, a mask is
more important.
“With our social distancing, wearing of the
mask, the data’s already showing that it’s been
effective. It’s, you know, cost savings, and
it’s effective. And it can go a long way,” said
Brennan, who oversees multiple clinical trials
using drugs such as Remdesivir, which President
Donald Trump received on Oct. 2, to treat
Covid-19.
Dr. David Ho, a world renowned
virologist working on developing monoclonal
antibody therapies for Covid-19 at Columbia
University, who also appeared on “60 Minutes,”
emphasizes the importance of masks too. He
tells CNBC Make It that “masks are key right
now.” But “we need as many tools in our tool box
as we can have in dealing with this pandemic,”
says Ho, and “vaccines and therapies would make
a huge difference as well.”
Dr. Kevin Tracey, who spoke to “60 Minutes” and
heads the Feinstein Institutes for Medical
Research at Northwell Health, says masks are key
in tackling Covid-19 now.
“The reality is we know the masks work,” Tracey,
who is doing clinical trials and research around
Covid-19 treatments, tells Make It. But also,
“we know we need to make therapies and
vaccines.”
It is puzzling that so little information is
being published about mask leakage and mask
efficiency. Distinguished professionals are
using the term mask to indicate some product
much more specific than just a “pill” but the
public needs to be made aware of the big
differences in mask quality.
FDA will No Longer Review Chinese Masks for
Addition to the Approved List
Today, October 15, the U.S. Food and Drug
Administration (FDA) reissued the Emergency
Use Authorization (EUA)
for certain filtering face-piece respirators (FFRs)
that are manufactured in China and are not
approved by the Centers for Disease Control and
Prevention’s (CDC) National Institute for
Occupational Safety and Health (NIOSH).
Under the June 6, 2020 version of this EUA, a
respirator was authorized if it met any of three
predetermined eligibility criteria. Effective
immediately, the reissued EUA no longer includes
the three eligibility criteria, meaning the FDA
will no longer review requests nor add to the
list of authorized respirators–known as Appendix
A—of this EUA based on those criteria.
The FDA recognizes there is still a shortage of
FFRs, and to provide additional capacity as
needed, the agency is continuing the emergency
use authorization of respirator models that are
already included in Appendix A of this reissued
EUA.
“Since the beginning of the COVID-19 public
health emergency, we have taken appropriate
actions to support the personal protective
equipment needs of our health care personnel by
issuing EUAs. As part of our continuing work to
meet the demands of this public health
emergency, we undertook and completed a shortage
assessment and concluded that reissuing this EUA
was appropriate to reflect the current U.S.
demand for these products,” said Suzanne
Schwartz, M.D. M.B.A., Director of the FDA’s
Office of Strategic Partnerships and Technology
Innovation in the Center for Devices and
Radiological Health.
To further inform the EUAs, the FDA completed a
respirator shortage assessment to understand
current product availability for both
NIOSH-approved N95s and KN95 respirators and use
practices for each. The assessment shows that
the KN95 respirator models authorized by this
EUA meet the demand for these respirators. As
part of this assessment, the agency heard
directly from health care personnel that the
KN95 design has limited adoption in health care
settings; from distributors that imported,
non-NIOSH-approved product from China is sitting
in warehouses unused; and from manufacturers
that NIOSH-approved N95 production is
increasing. Additionally, CDC/NIOSH continues to
issue more N95 approvals.
The FDA is reissuing this EUA to authorize only
those respirators the FDA had already authorized
and that are presently listed in Appendix A. As
outlined in the reissued EUA, FDA has removed
the previous eligibility criteria and,
therefore, no additional respirator models will
be added to Appendix A under those criteria. As
such, the FDA is no longer reviewing requests
submitted based on the June 6, 2020 EUA’s
criteria.
As a result of this EUA’s reissuance, FDA
expects that staff and agency resources that
were devoted to reviewing those submissions can
instead focus on other critical needs during the
COVID-19 public health emergency, including
continuing to work with CDC/NIOSH to help
facilitate the availability of respiratory
protection that meets the applicable standards
and demands of health care personnel. The FDA is
committed to refining our policies and
approaches as appropriate to further facilitate
the development and availability of these
devices for health care personnel.
Qualitative vs Quantitative Fit Tests
According to OSHA, “a ‘fit test’ tests the seal
between the N95 mask’s, or respirator's,
facepiece and your face.” It typically takes
15-20 minutes to complete and should be
performed when this type of mask is first used
and then at least annually. The purpose of the
fit test is to assure that the mask fits and
seals properly so potentially contaminated air
cannot leak into the mask and so hazardous
substances are kept out. The fit test must be
conducted using the same make, model and size of
mask that the worker will use on the job. Fit
testing with a different type of mask than the
one that will be used does not assure proper
protection. If the model of mask used for the
fit test does not properly fit, another make,
model, style, or size of mask must be tested
until one that fits properly has been
identified. Employers need to provide staff with
a reasonable selection of sizes and models to
try. Once the fit test is completed and the
wearer knows which mask fits best, he/she should
always use the one shown to be the right ‘fit’
or ‘size.’ That way, it can be replaced with
another mask with appropriate fit. Fit tests can
be qualitative or quantitative. In dentistry,
the qualitative test is most often used.
Qualitative fit testing is normally used for
half-mask respirators like the N95, which cover
only the user’s mouth and nose. Qualitative fit
tests operate on a pass/fail method and do not
measure the actual amount of leakage. They rely
on the user’s sense of taste or smell, or the
person’s reaction to an irritant, to detect
leakage. The mask fails the test if the wearer
can detect any leakage of the test substance.
OSHA has accepted four qualitative fit test
methods:
1. Isoamyl acetate, which smells like bananas;
2. Saccharin, which leaves a sweet taste in your
mouth;
3. Bitrex, which leaves a bitter taste in your
mouth;
4. Irritant smoke, which causes coughing.
Information on quantitative fit testing can be
found on OSHA’s website. The agency’s resource,
Appendix A to §1910.134—Fit Testing Procedures
(Mandatory): Part I. OSHA-Accepted Fit Test
Protocols: A. Fit Testing Procedures—General
Requirements, offers helpful guidance. Mask fit
should be reevaluated any time the wearer
experiences changes in his/her physical
condition that could affect the fit. These
include: a significant change in weight (either
loss or gain); major dental work, such as new
dentures; facial surgery that changes the shape
of the face; or significant scarring in the area
around the seal. Employers must ensure that the
fit testing and recordkeeping requirements of
OSHA's respiratory protection standard are met
before staff can use a N95 mask for protection
against hazardous exposures at work. Employers
may allow personnel to use their own respirators
but cannot require them to do so.
Polymer Face Masks Developed at Ag School and
Being Tested at Meat-Processing Plants
While many people are encouraged and even
mandated to wear face masks to prevent the
spread of COVID-19, some complaints have become
common: The mask doesn’t fit correctly. It’s
uncomfortable. It’s too hot, or it’s hard to
breathe through.
A material scientist at The Ohio State
University (OSU) College of Food, Agricultural,
and Environmental Sciences (CFAES) is working to
change that.
Judit Puskas is in the final stages of
developing a polymer face mask she expects will
be more effective in the fight against COVID-19.
Puskas, who is a distinguished professor in
polymer science in the CFAES Department of Food,
Agricultural and Biological Engineering, has a
provisional patent application pending for the
mask she is developing.
She’s working with the Mayo Clinic to create and
test the mask to meet the same safety and
efficacy standards of an N95 mask, but with more
comfort and usability for the wearers. Puskas’
mask is made of a nonwoven fabric composed of
biocompatible rubber composite formed into a
fiber mat that can be used to create personal
protective equipment, including face masks.
The goal, she says, is to offer alternatives to
the current market of N95 masks that can be used
by workers in a wider variety of conditions and
situations.
“The current N95 masks protect against the
virus, but most people say the masks aren’t
comfortable and aren’t easily breathable,”
Puskas says. “There are other polymer fiber mats
used in N95-equivalent masks, but they are based
on rigid plastics and don’t offer much
flexibility.
“The material I’ve developed is a flexible,
breathable rubber, that can be made into
comfortable-fitting masks. Additionally, it’s
water-repellant, doesn’t allow sneeze particles
through, nor will it let moisture build up on
the mask from breathing. This rubber can also be
used in hot, humid conditions as well as in a
freezer, can be easily sterilized in water-based
solutions, and is recyclable for multiple
reuse.”
It’s for those reasons that Puskas’ masks, once
completed, will be tested by more than 100
workers in five meat-processing plants across
Ohio to gauge the masks’ effectiveness for ag
workers in hot, humid conditions.
CFAES researchers, including Lyda Garcia, an
assistant professor of meat science; Mary
Rodriguez, assistant professor of community
leadership; and Joy Rumble, assistant professor
of agricultural communication, are working with
meat processors statewide to determine the
barriers workers face when considering masks as
personal protective equipment to reduce the
potential spread of COVID-19.
The team is also researching workers’
perceptions about wearing personal protective
equipment to better understand why some choose
not to wear it. The team’s goal is to develop
strategies to get more workers to change their
perception of masks and to choose to wear them,
Rodriguez says.
According to the Centers for Disease Control and
Prevention (CDC), the meat and poultry
processing industry, which is an essential
component of the U.S. food infrastructure,
employs approximately 500,000 people nationwide,
many of whom work in close proximity to other
workers, often in hot, humid conditions.
Ohio meat processors reached out to Garcia, who
is also an Ohio State University Extension meat
science specialist, and her team for help in
determining solutions for personal protective
equipment, including understanding what works
and how to encourage workers to wear personal
protective equipment such as face masks, Garcia
says. OSU Extension is the outreach arm of
CFAES.
Garcia says the conversations with meat
processors helped her team realize that
understanding employee behavior and attitudes
about wearing personal protective equipment such
as masks would be valuable in helping Puskas
modify and adjust her mask design.
“Understanding employee behavior, attitudes and
leadership is critical when designing personal
protective equipment,” she says. “The most
common complaint among workers is that masks are
uncomfortable, tend to move and slip off, and
they have to keep adjusting them.
“Another drawback is that workers say it’s
difficult to breathe in masks in hot, humid
conditions while constantly moving, and they
don’t like breathing in their own carbon dioxide
that is trapped in the masks in those
conditions.”
Additionally, Garcia says, with the fans and the
air conditioning noise in the plants, workers
say wearing masks makes it harder to communicate
with each other, which could be a safety issue
considering the sharp knives and tools they use.
“Also, these workers are on their feet 8 hours a
day, wearing hair nets, safety glasses, with
some men having long beards, then you add a mask
and you can see where things can become more
complicated fast on top of just wearing the
mask,” she says. “At the end of the day, it’s
about working with employees to encourage them
to wear personal protective equipment.
“Not only will it serve to protect the food, but
it will help keep the employees safe and
healthy, and, in turn, will keep them working to
provide a wholesome, sound, high-quality
product.”
That’s one of many reasons why Puskas was
compelled to develop a mask using the
biocompatible rubber composite fiber mat she
created as a way to add comfort, flexibility and
breathability to the N95 mask market.
http://www.angusbeefbulletin.com/extra/2020/08aug20/0820fp_B_FaceMasks.html
How Efficient are Masks Once Leakage is
Included?
It is interesting that McIlvaine found better
information on leakage for masks worn by the
public in air pollution
research rather than COVID. There is a
good analysis on the Smart Air website dating
back three years.
Thomas is an Associate Professor of Behavior
Science at the University of Chicago Booth
School of Business and the founder of Smart Air,
a social enterprise to help people across the
world breathe clean air without shelling out
thousands of dollars for expensive purifiers.
In addressing the mask leakage Talhelm said
“This question is tougher to answer because you
have to measure the mask while you’re actually
wearing it. For that, you need a really
expensive fit
test machine.
Fortunately, I begged and begged 3M until they
let me use their lab in Beijing.

The blue tube is sampling air outside the mask,
while the white tube is sampling air from inside
the mask.
Smart Air co-founder Anna Guo and Beijing-based
Dr. Richard Saint Cyr also
tested masks,
so I combined all of our data.
The 3M masks consistently performed the best in
these tests. The Vogmask performed fairly well,
capturing 95% of pollutants. Big-name masks like
the Respro and Totobobo masks both captured less
than 85% of pollutants.
It’s important to make clear: masks that fit my
face well might not fit other people’s faces
well. However, there is evidence from a broader
population that masks fit most people well. A
scientific study of 3M models on 22 Chinese
people found a median fit score of
99.5%–essentially the same as the top results
from Dr. Saint Cyr and me.
Best yet, effective masks don’t cost a lot of
money.
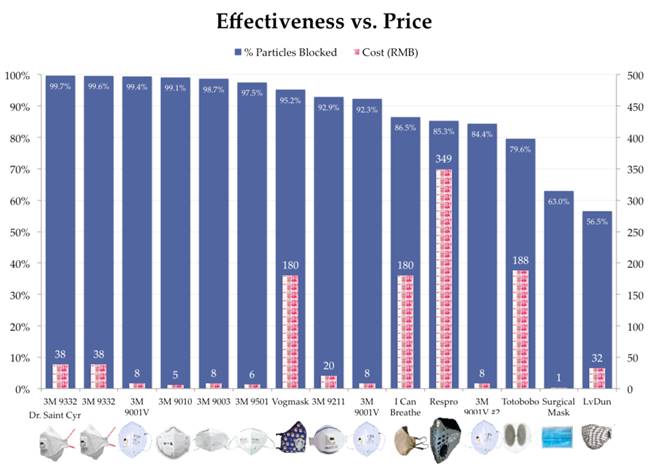
The branded masks – Vogmask, Respro, I Can
Breathe and Totobobo – all cost the most.
However these masks consistently performed worse
than cheaper 3M masks. Only the Vogmask was able
to capture over 95% of particulates (meeting the
N95 criteria).
OSHA will Levy Fines for Failure to Have
Appropriate Mask Fit Testing
Guy Burdick asks and answers the following
question.
Will workplace safety and health inspectors show
up at your facility if workers contract
coronavirus disease 2019 (COVID-19) and one of
them files a complaint? Yes; in fact, it’s
already happened.
Although there are no federal standards for
coronavirus exposure or airborne infectious
disease, COVID-19 has become a recognized
workplace health hazard since the pandemic was
declared in March. You can be cited for failing
to protect your employees from the coronavirus
under the General Duty Clause of the
Occupational Safety and Health (OSH) Act of
1970.
Section 5(a)(1) of the OSH Act reads: “Each
employer shall furnish to each of his employees
employment and a place of employment which are
free from recognized hazards that are causing or
are likely to cause death or serious physical
harm to his employees.”
The federal Occupational Safety and Health
Administration (OSHA) has cited several
employers under the General Duty Clause for
failing to protect their employees from the
coronavirus.
OSHA cited JBS Foods Inc. of Greeley,
Colorado—operating as the Swift Beef Company—for
failing to protect employees from exposure to
the coronavirus. The agency cited JBS Foods with
a violation of the General Duty Clause for
failing to provide a workplace free from
recognized hazards that can cause death or
serious harm and proposed penalties of $15,615,
the maximum allowed by law.
The agency conducted a coronavirus-related
inspection and also cited the company for
failing to provide an authorized employee
representative with injury and illness logs in a
timely manner following OSHA’s inspection.
The agency cited Smithfield Packaged Meats Corp.
in Sioux Falls, South Dakota, for a General Duty
Clause violation for failing to protect
employees from exposure to the coronavirus. OSHA
proposed a penalty of $13,494. At least 1,294
Smithfield workers contracted the coronavirus,
according to the agency, and 4 employees died
from the virus in spring 2020.
In addition to the General Duty Clause, the
agency also is enforcing all federal standards,
including the respiratory protection standard
despite ongoing shortages of N95 filtering
facepiece respirators.
OSHA cited Bergen New Bridge Medical Center for
respiratory protection violations at its
Paramus, New Jersey, location. The hospital
failed to fit test tight-fitting facepiece
respirators on employees who were required to
use them, train employees on proper respirator
use, and ensure employees understood when to
wear a respirator, according to OSHA. The agency
proposed penalties of $9,639.
OSHA cited Georgetown Dental LLC in Georgetown,
Massachusetts, for respiratory protection
violations, proposing penalties of $9,500. The
agency cited the dental practice for failing to
provide medical evaluations and initial fit
testing for employees required to wear N95
respirators as protection against the
coronavirus; a lack of written programs for
bloodborne pathogens (BBP), hazard
communication, and respiratory protection; and
insufficient bloodborne pathogen training and
controls and inadequate eyewash stations.
OSHA cited Hackensack Meridian Health for a
serious violation for failure to provide
respirators to resident-care employees at its
North Bergen, New Jersey, location in March.
Employees without respirators were caring for
residents who were exhibiting symptoms of
coronavirus infection, according to the agency.
OSHA proposed penalties totaling $28,070. Other
violations included failure to conduct
respirator fit testing, effective training, and
compliant medical evaluations for the period
after the employer began providing respirators
to the employees and requiring their use.
The agency cited Ohio-based healthcare provider
OHNH EMP LLC for violations of the respiratory
protection standard after the company reported
the coronavirus-related hospitalization of seven
employees. The agency proposed penalties
totaling $40,482. The agency also issued the
company a Hazard Alert Letter regarding the
company’s practice of allowing N95 respirator
use for up to seven days or until damaged or
soiled. The company also had instructed
employees to wear surgical masks issued each day
over their respirators. Wearing a surgical mask
over a respirator could interfere with the
respirator’s inhalation and exhalation
resistance—factors the National Institute for
Occupational Safety and Health (NIOSH) tests
respirators for in addition to particle
filtration when certifying respirators.
The agency cited three of the company’s
locations for serious violations of the
respiratory protection standard’s requirements,
including lack of a comprehensive written
respiratory protection program and medical
evaluations of employees provided with and
instructed to wear respirators.
State workplace safety and health agencies also
have cited employers for inadequate COVID-19
protections. Governors in Nevada and Oregon have
ordered their workplace safety agencies to
ensure employers’ compliance with their states’
COVID-19 restrictions in addition to enforcing
their states’ occupational safety and health
laws. California and Michigan have cited several
employers for coronavirus-related violations.
The California Division of Occupational Safety
and Health (Cal/OSHA) announced it had cited 11
employers in agriculture, food processing,
health care, meatpacking, and retail for not
protecting employees from coronavirus exposure.
Cal/OSHA conducted a complaint-initiated
inspection of DL Poultry, Inc., of Monterey Park
and proposed penalties of $51,190 for COVID-19
and other violations. The agency proposed
penalties of $9,000 following a
complaint-initiated inspection of Olson Meat
Company, a meatpacking facility in Orland,
finding the employer did not physically distance
employees at least 6 feet apart in processing
areas or install plexiglass or other barriers
between workers.
The agency cited a frozen food manufacturer and
a temporary employment agency for failing to
protect hundreds of employees from COVID-19 at
two frozen food plants. The employers did not
implement procedures to have employees work at
least 6 feet away from each other or install
barriers and did not investigate employees’
COVID-19 infections that included more than 20
illnesses and 1 death.
The agency has cited agricultural labor firms
with COVID-19 and other violations, including:
-
Michel Labor Services Inc. for
COVID-19 and heat illness
prevention violations and
proposed penalties of $11,700
following an inspection of a
Dixon worksite; and
-
Serve Max Farm Labor Contractor
with both COVID-19 and heat
illness prevention violations
after an enforcement task force
inspection of a Vacaville
agricultural worksite, seeking
penalties of $11,250.
California also has an airborne transmissible
disease (ATD) standard that applies to
healthcare facilities, as well as correctional
facilities, diagnostic laboratories, and police
and public health services. Under the ATD
standard, California employers must protect
workers at healthcare facilities and other
services and operations from airborne diseases
like COVID-19 and tuberculosis (TB), influenza,
and pertussis (whooping cough).
Since the pandemic began, Cal/OSHA has cited
several employers under the ATD standard,
including:
-
Gateway Care & Rehabilitation
Center, a skilled nursing
facility in Hayward, for
exposing nurses and housekeeping
workers to COVID-19 when it
failed to provide necessary
personal protective equipment
(PPE);
-
Santa Rosa Police Department for
failing to implement required
screening and referral
procedures for persons
exhibiting COVID-19 symptoms and
failing to report to Cal/OSHA
multiple serious illnesses
suffered by employees who
contracted COVID-19; and
-
Sutter Bay Hospitals’ CPMC
Davies Campus for not ensuring
its healthcare workers in
administrative medical offices
and security guards in the
emergency department wore
respiratory protection, as well
as medical staff without N95
masks or other proper protection
while performing a medical
procedure in the operating room
on a suspected COVID-19 patient.
The Michigan Occupational Safety and Health
Administration (MIOSHA) cited 19 businesses with
serious “general duty” violations for failing to
protect employees from coronavirus exposures.
Violations cited under the state’s reopening
guidelines included:
-
Lack of a preparedness and
response plan and failure to
designate a COVID-19 workplace
supervisor;
-
Failure to require face
coverings when social distance
could not be maintained;
-
Failure to train employees on
COVID-19 guidelines;
-
Failure to conduct a daily
health screening protocol and
maintain/retain documentation
for training, entry screening,
and contact tracing; and
-
Failure to post signs, markings,
and barriers at the time clock
and provide cleaning supplies
for high-touch surfaces.
https://ehsdailyadvisor.blr.com/2020/10/covid-19-enforcement-a-guide-for-ehs-professionals/
Vogmask is a Lightweight Mask with a Good Fit
Ethan Brooke is the person behind
BreatheSafeAir.com. He has addressed mask fit in
his blog and had the following observations
about the fit with Vogmask
It’s important to note that the filter
standards (N95, KF94, KN95, etc.) are irrelevant
if the mask isn’t fitted correctly. If there are
gaps in the seal, particles can simply enter
through these gaps rather than through the
filter.
For this reason, the fit of a mask is vital. For
the best fit, professional fit testing is
required. However, this is not accessible for
most people.
3M recently found that even without professional
fit testing that some level of protection can be
achieved if methods are followed to create a
good fit (3M).
If you are wondering how to don a respirator
properly, please refer to this guide by
the CCOHS.
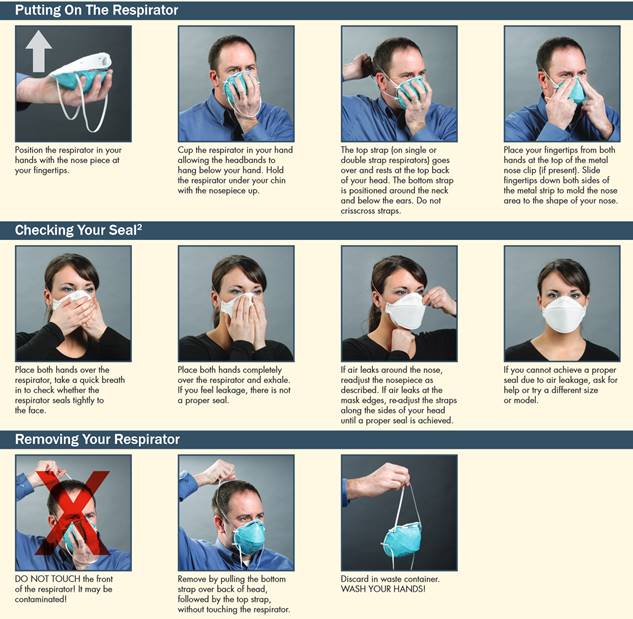
It is vital that a mask fits as well as possible
and that a seal can be made between your mask
and your face.
Vogmask offers five different sizes, each meant
for different people. The mask sizing is meant
to be easily accessible and is therefore based
on the height of the wearer. However, if you
have strong facial features, you may find
achieving a fit hard. This is especially true if
you have a big or small nose.
Vogmask uses ear-straps, and although common on
respirators, this is one of Brooke’s biggest
issues with the product. Ear-straps are less
sturdy than a head-band style of strap and mean
that the mask is more loosely fitting. Further,
these ear straps (as with all ear-straps) become
very painful after long periods.
Luckily, a headband accessory is available. If
you are purchasing a Vogmask and expect to be
wearing it for long periods of time, Brooke
can’t recommend this headband enough. Your ears
will thank you for it!
The mask features a wire-nosepiece for
adjustments around the nose. Although easily
adjustable, it’s important to try to retain the
structural integrity of the product when storing
it. This means, if possible, don’t share the
mask and don’t store it in the sun, or somewhere
where it can be crushed.
Another aspect that I noticed when first trying
Vogmask is that the mask is very light. It is
lighter than the Cambridge Pro Mask that I
Brooke usually uses, and it also feels
significantly lighter than most other reusable
masks that I use.
This is great for long-term wear. Although the
weight doesn’t seem to make a big difference
initially or in theory, in practice after long
periods of wear it will be a lot more
comfortable.


