
Coronavirus
Technology Solutions
October 8, 2020
Aerosols for HEPA Filter Testing
Austin Air Canada Struggling to Keep Up with
Demand for Air Purifiers
Delivery Dates of MERV 13 Filters Extended by
Two Months
Shortage of HVAC Filters Reported by Contractors
Fauci Says Death Toll Could Reach 400,000
Plexiglass Barriers at VP Debate Send the Wrong
Message in the COVID Battle
Freudenberg has Medical Face Mask with Soft
Three Layer Polypropylene Media
Thrace Invests in Surgical Masks and Needle
Punch Non Wovens
Silicon Nitrite Bonded to Mask Fibers
Inactivates COVID
Three Finnish Companies, Join Forces to Develop
and Start Production of Finnish, High-Quality
Respirators
IMSTec has Built Eleven Mask Manufacturing Lines
Bicma Machine can Make 800 Masks per
Minute
Feilong Supplies Up to 3.5 tons per day
of Meltblowns
COVID Viable for Nine Hours on Human Skin
______________________________________________________________________________
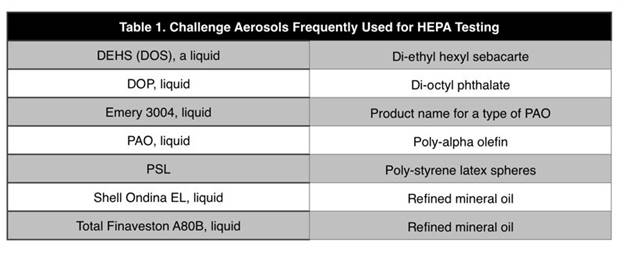
Aerosols for HEPA Filter Testing
HEPA filter testing upon installation and
periodically thereafter was addressed by Jesus
Casas in a Cleanroom Technology article.
Among the topics was the selection of
aerosols. There are three types of aerosol that
can be used for testing filter installations in
a cleanroom. These are:
-
Cold generated aerosol
-
Hot generated aerosol
-
Microspheres
The first two are formed from oil-type liquids.
Polystyrene latex (PSL) spheres are most
commonly used in microelectronic applications,
while polyalphaolefin (PAO) in life sciences.
The information in Table 1 describes the typical
challenge aerosols used for HEPA testing.
Before starting the filter scan, it is necessary
to set the concentration of test aerosol
particles upstream of the filter. The ISO
14644-3 standard suggests a concentration
ranging 10µg/l and 100µg/l should be used for
the photometry test method. It also suggests
that concentrations lower than 20µg/l reduces
sensitivity, and concentrations over 80µg/l give
filter fouling. It is best to use the lower
recommended concentration to minimize the
potential for blockage or a bleed
-through event.
Austin Air Canada Struggling to Keep Up with
Demand for Air Purifiers
A Waterloo-based company says it's sold more
than 6,000 HEPA air purifier units to schools
across Ontario, and it's struggling to keep up
with surging demand caused by the COVID-19
pandemic.
"The shortage is very, very serious," said Alex
Taylor, president of Austin Air Canada. "But
it'll be dramatically worse once everyone moves
inside."
The devices, which are medical grade HEPA air
filters, reduce the viral load of COVID-19 in a
classroom, said Taylor. More than 15 school
boards across Ontario have purchased units from
Austin Air Canada, and "every day, more and more
are direct contacting us trying to get units."
A spokesperson for the Waterloo Catholic
District School Board said it had purchased
1,100 units. The Upper Grand District School
Board is also a customer, said Austin Air.
Austin Air is one of the few that produces its
own filters and units at a 480,000 square foot
facility in Buffalo, New York, and doesn't rely
on any other countries to manufacture its
products.

The company is busy filling orders from more
than 15 Ontario school boards, it says on its
website its models appropriate for dealing with
COVID-19 come with HEPA and carbon cloth or
granular carbon filters.
But the surge in demand has Austin Air Canada
considering, for the first time, outsourcing
production to a Canadian partner so that the
U.S. facility, which is part of a separate
corporate entity, can focus on demand in America
and other parts of the world.
Taylor said the backup strategy would ramp up
production in Canada "quite quickly" if needed.
Right now, the manufacturing facility is
producing 1,000 units a week for Canada.
Interest for the units isn't just coming from
school boards. Austin Air has supplied thousands
of units to dentist and doctors' officers over
the past few months, said Taylor, and he's
expecting to see demand from restaurants,
post-secondary institutions and long-term care
facilities in the future too.
-
"We tripled our capacity and
we're still just able to meet
demand," he said. The company
has sold more than 6,000 units
to more than 15 Ontario school
boards at the time of the
interview.
The company has stopped selling to partners in
Europe and Asia. Even so, Taylor said "we'll be
maxed out within a month."
Delivery Dates of MERV 13 Filters Extended by
Two Months
Some HVAC industry leaders warn that supplies of
the recommended filters, known as MERV-13, are
insufficient to meet mounting demand—and will be
for the foreseeable future.
“We’re 60 days out on our MERV-13 supply,” says
Danny Miller, president of HVAC engineering firm
Transformative Wave. “[If] this moves from a
recommendation from the CDC to a regulatory
requirement, frankly these guys are screwed,
because the supply doesn’t exist right now. This
is going to be a crisis.”
“There’s no way,” Rob Castor, vice president of
sales for air filter manufacturer AAF
International, says of the possibility of
rapidly upgrading filters on air-handling
systems nationwide. “It’s not going to happen.”
Under normal conditions, the higher-density
MERV-13 filters are in demand only from
industrial or medical facilities with specific
air quality concerns, making up just about 5% of
HVAC filter demand. Demand has increased by as
much as 10 times, experts estimate. But filter
supply is unlikely to keep up because of the
high cost of increasing production capacity for
what could be a short-term demand spike.
Terry Ritchie, national sales lead for filter
manufacturer Trion, says wait times for MERV-13
filters ordered from his company have roughly
doubled, from two weeks to four weeks, and he is
confident the delays will get longer. Grainger,
a major industrial supply wholesaler, reports
that it has completely depleted its stock of
MERV-13 filters on hand due to pandemic-driven
demand.
Castor says some filter manufacturers have
already begun “allocating” stock or only
partially filling orders in order to distribute
supply more evenly. He says AAF may have to
begin doing the same soon.
Filter manufacturers say they are doing
everything in their power, including running
double shifts at factories, to stave off
shortages. But manufacturers told Fortune that
the main constraint on MERV-13 supplies is a
precursor material known as filter media.
Machinery for creating MERV-13-class filter
media is very costly, making major capacity
increases financially risky for the firms that
produce it, since the current demand surge may
prove temporary.
Filter media producers sell their product in
bulk to companies such as AAF who turn it into
HVAC filters. But the media is also used for
emergency respirators and for the masks that
have become commonplace in the pandemic era.
Some industry leaders say the increased demand
for MERV-13 media for use in masks and
respirators has exacerbated the shortage for
HVAC filters.
Shortage of HVAC Filters Reported by Contractors
HVAC filters are emerging as a crucial element
in the efforts to reopen buildings such as malls
and schools. The heightened and unexpected
demand creates questions about supply.
In some cases, governments are mandating filter
upgrades. New York Gov. Andrew Cuomo at one
point said all malls in his state must install
HEPA filters before allowing in the public.
Cuomo revised the rule so that shopping malls of
800,000 square feet or more must have filters
with MERV 13 ratings or better. If a property’s
HVAC operation met other “enhanced” protective
criteria, then MERV 11 filters could suffice.
Even without mandates, guidelines for re-opening
buildings often include filter upgrades. Many
industries face supply issues right now, but
some filter manufacturers say their industry is
ready. Mike Rimrodt, vice president of marketing
at Aprilaire, said his firm is prepared to meet
this demand.
“We have no concerns meeting the current
increase in demand,” Rimrodt said. “We’ve
committed the capital investment necessary to
address capacity issues and do not foresee major
shortages given the current state. We have a
strong supplier base, and we have no issues
sourcing needed equipment to add capacity.”
Rimrodt did say the company’s supplier does have
some limitations on MERV 16 availability, but he
said Aprilaire executives still believe they can
meet demand.
Aprilaire may be meeting demand, but contractors
say there is a shortage. One contractor posted
online that he works with four suppliers for
filters and they all have a lead time of at
least 12 weeks for MERV 13 filters. Neil Smith,
founder of HVACQuick, said it took three months
for his supplier to deliver the HEPA filter
boxes his company needed for a government
contract. The supplier said they would have them
in three weeks.
Rich Morgan, CEO of Magic Touch Mechanical Inc.
in Mesa, Arizona, said he started to notice a
shortage of MERV 11 and 16 filters starting in
May. Morgan said his suppliers are doing a good
job of keeping him informed about what is in
stock in Arizona and what to expect. This keeps
the firm from having long backorders, he said.
Morgan said more clients are choosing Magic
Touch’s “Clean Air” add-on options when
purchasing replacement equipment, increasing
demand for higher-end filters. Magic Touch uses
its customer management system to schedule
reminders for clients due for filter changes.
Morgan said the firm now sends those reminders
sooner in attempt to head off an emergency call.
“We’re also informing our clients on the
shortages the industry is seeing and encouraging
them not to procrastinate until it becomes a
problem,” Morgan said. “We’ve been using both
our blog and social media to spread that message
as well — not only on air filter shortages, but
equipment and parts shortages as well.”
Roger Mariusso, sales and service manager at All
Makes Heating & Air Conditioning Corp. (AMHAC)
in Eastchester, New York, said he sees a delay,
especially for higher grade filters. Mariusso
doesn’t call it a shortage, because it’s only
delayed by a couple of weeks. Still, AMHAC
increased its orders for all equipment to ensure
availability.
“It’s not something that’s sensitive because we
keep a good stock here to dodge that kind of
thing,” Mariusso said.
As in most part of the country, demand for all
types of HVAC services boomed in suburban New
York once the weather grew warmer. Consumers are
looking for ways to keep safe, he said. They
want everything from filters to UV lights to
dehumidifiers.
“Indoor air quality has become a must for every
sale,” Mariusso said. “A year ago, I didn’t
bother to offer it to many customers.”
Most higher-MERV filters use electrostatically
charged meltblown synthetics, the same as N-95
masks. This means the filters being prescribed
for buildings and the masks required for
hospitals are competing with each other. Joe
Gorman, product manager for comfort air products
at Camfil, said the demand for filters will only
continue as building operators install
higher-grade filters. A MERV 13 filter loses its
charge over time, becoming in fact a MERV 7 or 8
filter. This means cycling through more filters
to meet the efficiency standards. Camfil
promotes MERV 13A, which Gorman said will
sustain a level of protection for a longer time,
meaning fewer changeouts.
“If you’re staying in the same footprint and
you’re going to a higher efficiency, your
filters going to come to the end of its life a
lot sooner,” Gorman said. “You’ve got to look at
the efficiency of the filter over its life, not
just on day one.”
There are other media used to make filters. A
main alternative for meltblown synthetics is
fiberglass. Kim Hager, Winsupply's HVAC hydronic
product marketing manager, said these filters
were becoming hard to find last year. Suppliers
had been moving away from these filters, Hager
said, because the profit on fiberglass was too
small for many providers. Now they are
reconsidering that decision.
Hager said suppliers are definitely seeing
strong demand for filters of all types. She said
one told her their gross sales were up more than
200 percent in June. Keeping up with that demand
has been challenging, especially with the
staffing difficulties brought on by the
coronavirus pandemic. Despite these challenges,
Hager said the industry effort to keep up with
demand is impressive.
“They’re working really hard to get caught back
up, and they’re doing a really good job,” she
said.
https://www.achrnews.com/articles/143648-hvac-contractors-find-filters-in-short-supply
Fauci Says Death Toll Could Reach 400,000
Anthony Fauci, the nation's leading infectious
diseases expert, said Tuesday that as many as
400,000 Americans could die from COVID-19 if
action isn't taken in the fall and winter.
Fauci told attendees of a virtual event held by
American University that between 300,000 and
400,000 could die from coronavirus in the
country.
"The models tell us if we don't do what we need
to in the fall and winter, we could have
300,000-400,000 COVID-19 deaths," American
University quoted the director of the National
Institute of Allergy and Infectious Diseases as
saying
Fauci's prediction goes beyond a University of
Washington study from August that said as many
as 300,000 people could die of COVID-19 by Dec.
1.
As of Wednesday morning, the U.S. has recorded
210,918 deaths and more than 7.5 million
confirmed infections of COVID-19, according to
data from Johns Hopkins University.
Fauci also asserted on Tuesday that a vaccine
will probably not be available to most Americans
until next summer or the fall, aligning with
Centers for Disease Control and Prevention
Director Robert Redfield's Senate testimony last
month. Fauci said during an event on Monday that
this means life may not return to normal until
the end of next year
Plexiglass Barriers at VP Debate Send the Wrong
Message in the COVID Battle
Vice President Mike Pence and Sen. Kamala Harris
were seated more than 12 feet apart and
separated by two plexiglass barriers. But those
barriers are "entirely symbolic," according to
Dr. Bill Schaffner, an epidemiologist at
Vanderbilt University.
A person familiar with the debate planning told
NBC News that Harris' campaign asked for the
plexiglass to be used at the event at the
University of Utah in Salt Lake City.
The plexiglass is "minimal protection,"
Schaffner said in a phone interview, adding that
the barriers are mostly "cosmetic."
However, he added that barriers are one part of
a "layered approach" that includes testing and
distancing of everyone on stage. Those in the
debate hall are required to wear a mask and
there will be no handshake or physical greeting
between Pence and Harris, according to the
commission. Altogether, he said, the steps have
likely reduced the risk of spread occurring.
The plexiglass barriers are just one "part of
the CPD's overall approach to health and
safety," according to a fact sheet distributed
by the commission.
The debate took place indoors and, of course,
plenty of talking was expected. That's important
because the CDC released new guidance on
Monday that said the virus can spread through
particles in the air between people who are
further than six feet apart in certain
environments. The CDC said the risk of that
occurring increases indoors and when people are
doing certain activities, including speaking.
Jeff Siegel, a professor of civil engineering at
the University of Toronto and a specialist in
indoor air quality, ventilation and filtration,
said the risk of virus-carrying particles going
airborne in an environment like a debate when
people are talking loudly is "huge."
"On the plus side, it's a pretty big space,
so there's a big dilution effect," he said over
the phone, adding that Harris, Pence and the
moderator, Susan Page, will be spaced out
appropriately. The high ceiling and large room
will also help to reduce risk, he said.
"But they're not addressing things like
ventilation," Siegel said, adding that he hopes
the debate hall has appropriately up-to-date air
filtration and ventilation systems. "If I was
Vice President Pence's staff or Harris' staff, I
would certainly want to get a portable HEPA
filter in there."
The commission did not return CNBC's request for
comment on the building's filtration and
ventilation system.
Kimberly Prather, a distinguished professor of
atmospheric chemistry at the University of
California at San Diego, said that if Pence is
infectious right now, the risk of him spreading
it to Harris will increase as the debate goes
on.
"Imagine being in a room with one smoker,"
Prather, who has briefed White House coronavirus
advisor Dr. Anthony Fauci on the risk of
airborne spread, said in a phone interview.
"Over time, the longer you sit in that room, you
just see the haze build up."
Prather explained that the risk of airborne
spread is due to virus-carrying aerosols, very
small particles that can remain suspended in the
air and even travel by currents. The CDC
maintains that most transmission occurs through
larger respiratory droplets, but Prather has
long warned of the risk of aerosol spread.
"The louder you talk, the more you produce," she
said of aerosols, adding that without proper
ventilation, those plexiglass barriers won't do
much to stop the particles. "When I saw it I
laughed, but it's not funny."
Prather added that she and her colleagues are
concerned about what kind of message the
barriers send to the public. She said people and
businesses should focus more on air filtration
and ventilation than on erecting barriers,
though the two strategies can be implemented
together. Prather hopes that Harris and Pence
opt to wear a mask throughout the debate.
"There's no reason not to," she said.
Jose-Luis Jimenez, a chemistry professor at the
University of Colorado at Boulder who studies
how aerosols spread the virus, shared Prather's
concern about what message this sends to the
public. He said the debate should be moved
outdoors to send the message that "every
activity that can be moved outdoors, should be
done outdoors, with enough distance."
"Perhaps the ventilation rate is very high in
that debate hall, and thus the risk to the
debate participants is low. But they are sending
the message to millions of people that talking
indoors without a mask is safe, as long as
enough distance is kept," he said in an email.
"Those barriers are a joke. It is just theater,
to make it look like they are taking some
precautions."
Freudenberg has Medical Face Mask with Soft
Three Layer Polypropylene Media
Freudenberg Filtration Technologies has
developed a high-quality mask that has now been
certified as a TYPE II medical face mask (EN
14683). It combines effective health protection
with first-class wearing comfort. Freudenberg
has completed all the necessary certifications
within just a few weeks. Companies are able to
purchase large quantities from the middle of
October.
“At the heart of our medical face masks is a
soft three-layer polypropylene filter medium
that is characterized by its superior
breathability,” explains Dr. Thomas Caesar,
director Global Filter Engineering Industrial
Filtration.
To effectively protect exposed individuals, the
special structure of the three layers reduces
the release of aerosols contaminated with
viruses and bacteria. External laboratory tests
confirm a filtration efficiency of more than 98%
in aerosol separation.
The soft nonwoven fabric also offers optimum
wearing comfort and facilitates easy breathing.
The independent dermatological institute
Dermatest has rated the product's skin
compatibility as "excellent." Elastic ear loops
and a nose clip allow Freudenberg Filtration
Technologies' mouth-nose protection to be
individually adjusted and ensure a comfortable
fit. This complete “Made in Germany” package
makes the masks a comfortable companion in
everyday working life.
An important factor for the paint industry is
that the medical face masks are LABS-compliant
in accordance with standard 24364 (test class B)
of the German Engineering Federation (VDMA).
This means that employees can safely wear them
even in industrial painting environments, as
they do not cause contamination with substances
that impair paint wetting (LABS).
Freudenberg Filtration Technologies only
supplies its certified face mask to companies
that order 7,500 units or more. For small
household quantities, Freudenberg Home and
Cleaning Solutions also sells the medical face
masks under the Collectex brand to consumers and
companies from mid of October.
Thrace Invests in Surgical Masks and Needle
Punch Non Wovens
Thrace Group
has implemented a number of ad-hoc investments
in the production of Type I, Type II and Type
IIR surgical masks in the group’s facilities in
Xanthi, Greece, in Forfar, Scotland and in
Clara, Ireland. The total investment cost
amounted to €3 million.
The investment plan was completed with great
success and in a timely manner. The production
lines are now in operation, with a total
capacity of 1.5 million masks per day
approximately. Apart from the sales generated in
the Greek market, a large proportion of the
production capacity is channeled to the United
Kingdom and to the European Union.
The surgical masks produced are fully complied
with the relevant quality standards, while the
production is now performed through automated
and efficient processes, thus making the overall
process very competitive in financial terms,
utilizing the strong technical know-how and the
expertise developed.
It is noted that the investment plan included
the installation of production lines for ear
loops and nose wires, through which the group
has achieved a fully vertical integration of its
operations.
Two Needlepunch Nonwoven production lines have
been added to the European manufacturing plants
of Thrace Group, consolidating its leading
position in the European market.
By significantly increasing their needle punch
production in Northern and Southern Europe
combined, Thrace Group aims to better serve its
increasing customer base, both in Europe and
abroad. Providing improved product
characteristics, better service and further
reducing lead times, the Group strengthens its
presence among the top providers for the
Geotextiles, Agro-textiles and Industrial
fabrics markets worldwide.
The state-of-the-art production lines are
strategically placed in Greece (Xanthi) and the
U.K. (Scotland), and incorporate all
technological advances in Nonwoven Needlepunch
manufacture.
Silicon Nitrite Bonded to Mask Fibers
Inactivates COVID
Sintx Technologies, an original equipment
manufacturer of silicon nitride ceramic for
medical and non-medical applications, announced
progress toward the manufacture of a
“catch-and-kill” mask that will inactivate
respiratory viruses. Exposure to silicon nitride
has been shown to neutralize several bacterial
species and viral strains.
The company said that it has successfully
dispersed and embedded silicon nitride particles
into nonwoven and woven fabric fibers.
Optimization of this process, fabric safety and
efficacy testing, and manufacturing scale-up are
the next steps beyond this critical milestone.
“By investing in our human resources and
equipment, we expedited this development
in-house, rather than relying on outside
parties,” says Dr. Bal, President, and CEO of
Sintx Technologies. “An electron image shows
silicon nitride particles preferentially bonded
to polypropylene fibers, at the micron level,
without stray particles. Fabrics containing
silicon nitride from this process will be tested
for their antiviral effect. Previous scientific
data have shown that silicon nitride strongly
inactivates SAR-CoV-2, the virus causing the
Covid-19 pandemic.”
“Initial process development activities have
shown promising results and we are hopeful that
we will soon have a mask that catches and kills
the coronavirus,” says Bruce Lorange, CEO of
O2Today, who has an agreement with Sintx to
develop and commercialize antimicrobial face
masks. “We are excited by the rapid progress we
are making and looking forward to sharing more
updates as we have them.”
“We believe that
the consumer market for a face mask capable of
inactivating bacteria and viruses extends well
beyond the present concerns about Covid-19,”
says Dr. Bal. “In densely-packed Asian cities,
for example, respiratory viruses and air
pollution are common health concerns, and
protective mask use existed even before
Covid-19. Similar concerns may endure in Western
countries as well, long after a Covid-19 vaccine
is freely available. We believe that our
antipathogenic masks and the underlying fabric
technology will have lasting consumer demand.”
Three Finnish Companies, Join Forces to Develop
and Start Production of Finnish, High-Quality
Respirators
Three Finnish companies, Suominen Corporation,
Screentec Oy and TrueMed Oy, joined forces to
develop and start production of Finnish,
high-quality respirators.
Finland, with no domestic face mask production
at the time, had difficulties sourcing
high-quality masks at the beginning of the
Covid-19 pandemic.
Suominen, a globally leading nonwovens producer,
was one of the companies that promptly reacted
to the difficult situation. In an
ultra-fast-tracked innovation process Suominen
and its partners developed a novel nonwoven,
Fibrella Shield, suitable for use in the
manufacturing of respirators. The innovative
nonwoven, developed in only a few months, has
passed the European Standard EN 14683:2019 Type
II requirements in terms of filtration
efficiency and pressure drop.
“At the same time that Suominen was developing
the new material, Screentec Oy – a renowned
producer of medical electrodes and human-machine
interfaces for demanding environments – decided
to start production of high-quality face masks
at its Oulu works. After an exceptionally fast
installation phase, the new production line was
ready by late summer.
“The common aim, a fully domestic supply chain
for high-quality face mask production, was a
natural starting point for our cooperation with
Suominen. The plan is that in the future we will
mainly use Suominen’s Fibrella Shield in our
face masks,” says Screentec’s CEO Antti
Tauriainen.
Suominen has been working with TrueMed Oy, an
innovative Finnish start-up, already earlier.
TrueMed has developed an AI and machine
vision-based non-additive solution that is used
to detect original and counterfeit medicines and
medical products.
The aim of the cooperation between the three
companies is to be able to confirm the
authenticity of the masks and the nonwoven used
in them – in this case, Fibrella Shield – and
thus to guarantee end-user safety. The
cooperation also aims to produce important
inventory information for the customer, for
example, information about expiration days or
how many masks that have been used.
“Determining if the product is genuine or a
counterfeit is done through our mobile phone
app, TrueMed Scanner. We provide Suominen an
identification process and mechanism that can
detect the product authenticity on the fiber
level of the nonwoven material. Suominen
Intelligent Nonwovens utilizes TrueMed’s
proprietary AI and machine vision platform. At
the same time, we can read all the necessary
codes and markings on the product and its
packaging,” explains TrueMed’s CEO Jyrki Berg.
Fibrella Shield not only provides excellent
protection, it is also comfortable and easy to
use.
“By combining the deep know-how of the three
companies, we are able to offer our customers
high-quality face masks with verifiable
authenticity as well as the ability to follow
the inventory data. In the future many of these
kind of technical innovations, for example
related to the end-users safety, smart supply
chain data, carbon footprint information and so
on, can be taken into use with Suominen
Intelligent Nonwovens. This very fast joint
development project was also funded by Business
Finland research and development funding,” says
Suominen’s CTO Markku Koivisto.
IMSTec has Built Eleven Mask Manufacturing Lines
IMSTec GmbH, based in Klein-Winternheim near Frankfurt, is a specialist in the development of customized manufacturing processes for the medical, pharmaceutical, cosmetics and precision control industries which this year has turned its focus to the development of facemask manufacturing machines with integrated inline quality control.
“We were asked by German officials to help with the supply of facemasks because in March and April there was a very severe shortage,” said the company’s general manager Edgar Mähringer-Kunz. “So we have so far set up eleven manufacturing lines here in Germany and developed independent products accordingly. Our BlueBec FFP2 facemask design is currently undergoing the validation process. To set up the lines and achieve certification and move to manufacturing in volume has been achieved in a very short time, because the demand is right now.”
Both the machine process and product, Mähringer-Kunz stressed, have been developed based on extensive FMEA (failure mode and effects analysis) from project planning through to commissioning.
“The machines are fully automated, with only the
loading and unloading carried out manually,
which is a key requirement for high labor cost
countries,” he said. “This allows five lines to
be overseen by a single operator. The lead time
from order to delivery of a machine is currently
between three and four months.”
Bicma Machine can Make 800 Masks per Minute
Bicma, based in Mayen, is a specialist in the design and manufacture of lines for diapers, femcare and incontinence products which since 2018 has belonged to Winkler and Dünnebier, which is itself part of the $3 billion Barry-Wehmiller Group.
The company has also developed its own facemask machine this year, the Auxilium FM, with the emphasis on high speed production and a number of simple and smart innovations that address potential shortages of individual mask components.
Typical mask-making machines, said the company’s business area manager Sven Weissörtel, have operating speeds of around 120 masks per minute and require three operating staff at work stations positioned at the beginning and end of the line.
“They have simple unwinders and no web guiding or web tension control, so the machine needs to stop for nonwoven roll changes,” he said.
In contrast, the Auxilium FM can produce 800 products a minute, based on Norm EN 14683 up to type IIR standard three layer nonwoven construction, with its essential meltblown core and integrated ear elastics and nose clip.
“This equates to a million products a day based
on three-shift operation, making it the most
productive mask machine in the world,”
Weissörtel said. “It can replace six typical low
output machines and can optionally be equipped
with a stacker and packaging solutions.”
In one small innovation that could make a significant difference, the ear loops for the masks are made of a standard nonwoven and Lycra and are integrated in line, as opposed to pre-made elastic strings which constitute a separate raw material need.
“This ensures there can be no shortages and the ear loops can be easily adapted to different face anatomies while being extremely comfortable and soft wearing,” Weissörtel said. “Similarly, the nose clip is a standard aluminum wire as opposed to a pre-fabricated flat ribbon material, and again applied in line with similar benefits.”
Bicma has its own lab machine which is available for material tests to assist in a customer’s mask certification processes, which can save valuable time to market.
German-made facemask machines are also eligible for the government’s current subvention programme which will provide funding of 40% of the cost of the machine, with an additional bonus of 10% if the end-product manufacturer commits to purchasing 70% of its raw materials within the EU.
“We have received many orders for the machine
throughout Europe and anticipate strong future
demand, especially from manufacturers taking
advantage of the government support,” Weissörtel
said. “Currently we should be able to deliver
new machines by November to December, with the
MMD certification process taking approximately
six months.”
Feilong Supplies Up to 3.5 tons per day of
Meltblowns
Changshu Feilong Nonwoven Machinery Co., Ltd. is
a leading enterprise specializing in the
development and production of spunlace nonwoven
equipment. It has a history
of more than 30 years.
In order to meet the current market demand for
epidemic prevention products, it has specially
developed P2 and P3 meltblown
cloth production lines. Proved by actual use:
the electret effect of the production line can
reach P2 and P3 standards,
the performance is quite stable, the production
capacity is high ( 2.5-3.5 tons/day),
and the operation is simple.

COVID Viable for Nine Hours on Human Skin
The new coronavirus can linger on human skin
much longer than flu viruses can, according to a
new study from researchers in Japan.
SARS-CoV-2, the virus that causes COVID-19,
remained viable on samples of human skin for
about nine hours, according to the study. In
contrast, a strain of the influenza A
virus (IAV) remained viable on human skin for
about two hours.
Fortunately, both viruses on skin were rapidly
inactivated with hand
sanitizer. The findings underscore the
importance of washing your hands or using
sanitizer to prevent the spread of COVID-19.
"This study shows that SARS-CoV-2 may have a
higher risk of contact transmission [i.e.
transmission from direct contact] than IAV
because the first is much more stable on human
skin [than the latter]" the authors wrote in
their paper, which was published online Oct. 3
in the journal Clinical
Infectious Diseases.
"These findings support the hypothesis that
proper hand hygiene is important for the
prevention of the spread of SARS-CoV-2."
Earlier in the pandemic, researchers in the U.S.
analyzed how long SARS-CoV-2 could last on
surfaces and found it remained viable on copper
surfaces for up to 4 hours, on cardboard for up
to 24 hours and on plastic and stainless steel
for up to 72 hours, Live
Science previously reported.
However, for ethical reasons, examining how long
the virus can last on human skin is more
complicated — you can't just put samples of a
potentially lethal virus on people's hands.
So for the new study, the researchers, from
Kyoto Prefectural University of Medicine in
Japan, created a skin model using samples of human
skin obtained from autopsies. The samples
were collected approximately one day after
death. The authors note that even 24 hours after
death, human skin can still be used for skin
grafts, meaning that it retains much of its
function for some time after death. Thus, the
collected samples could be a suitable model for
human skin, the authors argued.
Using their model, the authors found SARS-CoV-2
survived on the human skin samples for 9.04
hours, compared with 1.82 hours for the
influenza A virus. When these viruses were mixed
with mucus, to mimic the release of viral
particles in a cough or sneeze, SARS-CoV-2
lasted an even longer time, about 11 hours.
However, both viruses were inactivated on skin
15 seconds after using hand sanitizer that was
80% ethanol.
"Appropriate hand hygiene … leads to the quick
viral inactivation [of SARS-CoV-2] and may
reduce the high risk of contact infections," the
authors said.
The authors note that their study did not
consider the "infectious dose" of SARS-CoV-2,
that is, the quantity of virus particles needed
to give someone an infection from contact with
contaminated skin, and so future research should
also examine this question.
