
Coronavirus Technology Solutions
September 23, 2020
3M Analyzing Various Mask Decontamination Approaches
Importance of Wearing Masks for Air Travel
MSU Receives FDA Authorization to Decontaminate Reusable N95 Masks for Hospitals
Self-Adhesive N95 Mask has Several Unique Features
Fauci: 'Disturbing Number' of COVID-19 Patients have Heart Inflammation
Genetic Changes in COVID Could Make Long Term Immunity Less Likely
______________________________________________________________________________
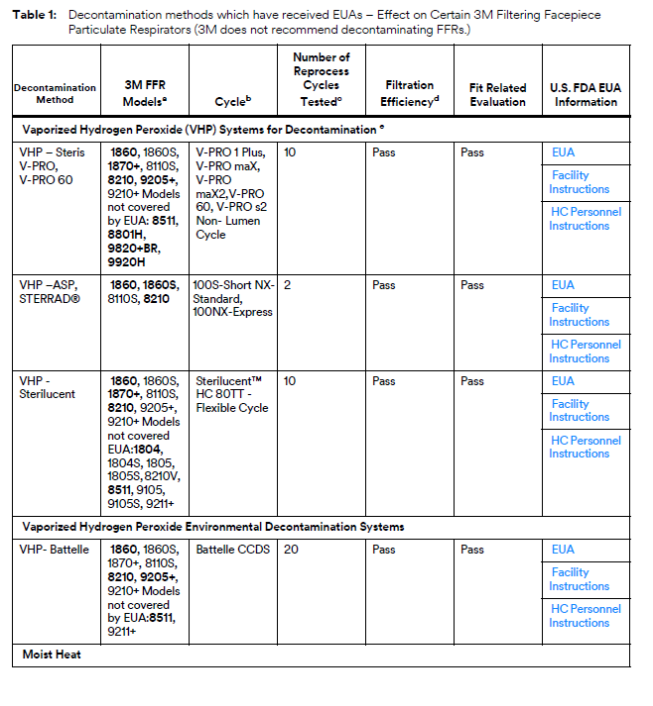
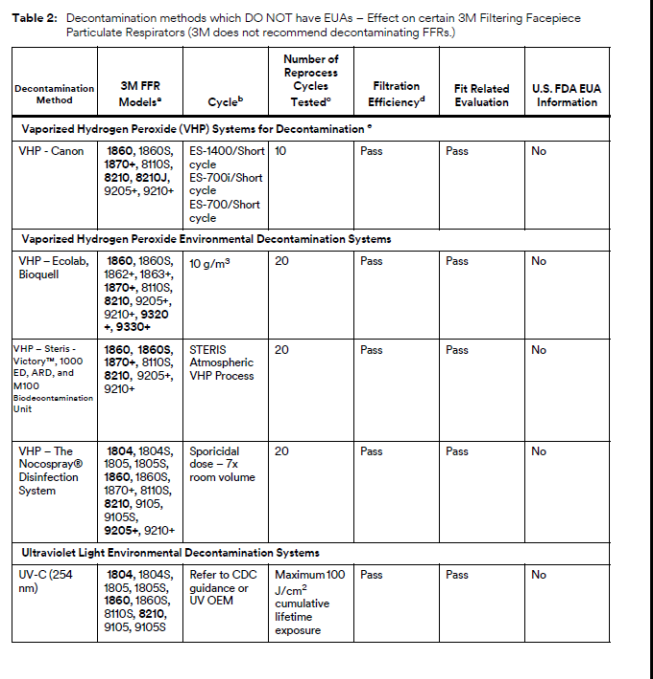
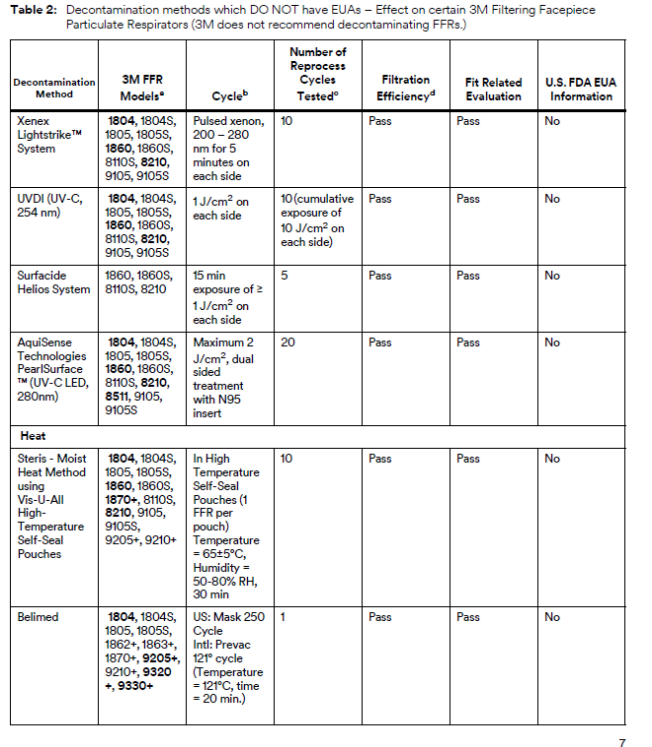
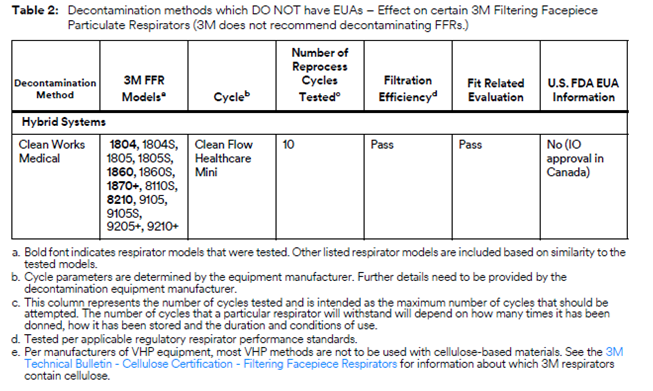
3M Analyzing Various Mask Decontamination Approaches
3M is collaborating with several equipment manufacturers and institutions that are investigating ways for hospitals to safely decontaminate 3M’s N95 FFRs in line with the CDC guidance on Decontamination and Reuse of Filtering Facepiece Respirators. 3M has been studying ways to decontaminate filtering facepiece respirators for years. There are at least four key aspects of successful decontamination of respirators. Many published studies do not take all four into consideration. The method must:
• inactivate the target organism, such as the virus that causes COVID-19;
• not damage the respirator’s filtration;
• not affect the respirator’s fit;
• and be safe for the person wearing the respirator.
If, as a result of decontaminating a respirator, the filtration is damaged or the respirator does not fit, it will not help reduce exposure to airborne particles at the level indicated, such as N95, FFP2, etc. In 3M’s work with external manufacturers of decontamination equipment, 3M relies upon the method developer to confirm the germicidal efficacy of the decontamination method and to provide information on potential hazards to the respirator user. 3M evaluates the effect of the method on filtration efficiency and integrity of their respiratory protection products.
To that effect, 3M is testing certain 3M N95 FFRs regarding the effect of the decontamination processes on fit and filtration performance. 3M is in the process of testing treated 3M respirators from multiple equipment manufacturers and institutions. Methods under evaluation include Vaporized Hydrogen Peroxide, UV, and Low Temperature Moist Heat, amongst others, as reflected in the CDC Guidance.
Other methods of decontamination are being discussed in public forums, including liquid chemical decontamination and time-based methods, but 3M is not prioritizing investigation of these methods at this time. Additional information about many decontamination methods can be found in the CDC guidance on Decontamination and Reuse of Filtering Facepiece Respirators, but again, many published studies have not considered all four key aspects listed above. 3M remains committed to providing data to the health care community as soon as possible.
Current information supports the following conclusions for all 3M filtering facepiece particulate respirators:
• 3M does not recommend the use of Ethylene Oxide or formaldehyde for respirator decontamination due to potential for repeat inhalation exposure to residual ethylene oxide or formaldehyde, known human airborne respiratory carcinogens. Ethylene oxide is an accepted sterilant for many device types, but given that the respirator is directly in line with a person’s breathing zone, it is not recommended for respirator decontamination.
• 3M does not recommend the use of Ionizing Radiation due to degradation in filter performance.
• 3M does not recommend the use of Microwave due to melting of the respirator near metal components resulting in compromise of fit.
• 3M does not recommend the use of High Temperatures above 75°C, such as Autoclave or Steam, unless specifically listed in the tables below due to significant filter degradation and fit degradation.
• 3M does not recommend the use of ethanol, isopropanol, quat solutions, soaps, or detergents due to degradation in filter performance.
• 3M does not recommend the use of Ozone due to degradation of headband and nose foam materials.
Importance of Wearing Masks for Air Travel
Harvard researchers (with funding from airlines, airports and other aviation interests) found that without a vaccine, the use of face coverings is “one of the most pragmatic and effective options” for controlling the spread of the disease in an aircraft – as long as every single passenger is masked up.
“Universal use is key,” the report said. “A recent modeling study suggests that the universal use of surgical masks in the setting of ventilation rates of aircraft may reduce infection risk from respiratory particles to less than 1 percent.” (Surgical masks are those lightweight, papery pale blue ones with horizontal pleats, commonly handed out by airlines and stores to customers who didn’t bring their own.)
The researchers note that earlier research conducted before the airlines adopted universal mask policies estimated an infection risk of 3.69%, although they note that some individuals may have picked up the virus before or after their flight.
“The use of face masks is critically important throughout the air travel process, from entering the airport for departure to leaving the destination airport, because it diminishes the release of infectious particles into the environment,” the report said.
What happens when passengers don't wear masks? Last week the CDC released results of a study that analyzed a "super spreader" event when one passenger in business class on a Vietnam Airlines flight in March, prior to universal mask wearing requirements, transmitted the disease to several nearby seatmates and flight attendants. Read about that flight here. Subsequent CDC evidence regarding transmission on planes is hindered by weak contact tracing in the U.S., so researchers can't be sure where transmission during air travel is happening, whether on planes, in airports, on public transportation, or on trips themselves.
MSU Receives FDA Authorization to Decontaminate Reusable N95 Masks for Hospitals
Vaporized hydrogen peroxide treatment modified by a Michigan State University veterinary team to decontaminate N95 masks for health care workers has been approved on an emergency basis by the Food and Drug Administration. The team is continuing to work with the FDA towards a September start of large-scale decontamination operations. MSU is the first and only public institution to receive this authorization to date.
When Columbus-based Battelle Memorial Institute received emergency use authorization for its VHP process in March, Gov. Gretchen Whitmer and MSU President Samuel L. Stanley Jr., M.D., tasked an effort that included the conversion of an animal housing area into a nine-room decontamination center now capable of cleansing tens of thousands of masks each week.
University Veterinarian F. Claire Hankenson, Director of Campus Animal Resources, is leading the project along with the MSU Office of Regulatory Affairs.
VHP was already in use at MSU sanitizing animal areas when the COVID-19 pandemic sparked widespread shortages of personal protective equipment for physicians, nurses and other medical personnel.
“We estimate that within our dedicated facility, running the VHP cycles five days a week, we can effectively decontaminate and redistribute approximately 14,000 masks per day (7,000 masks per cycle and two cycles per day) and up to 70,000 masks per week,” Hankenson said. “Over the coming fall and early winter, with a potential second surge of COVID-19 cases in the state (which may converge with influenza season), the MSU VHP decontamination process could ultimately recycle more than one million PPE devices back into the supply for the state of Michigan.”
Having received the FDA’s Emergency Use Authorization, MSU can expand its service beyond the original two partners in the pilot phase of the program: Sparrow Health System based in Lansing and Henry Ford Hospital System in Detroit.
“These health care partnerships will be essential for the successful execution of this project,” Hankenson said. “The Governor’s Office, the Attorney General’s Office and the office of Congresswoman Elissa Slotkin and others have all been heavily invested in the FDA EUA application and share in the success of this approval.”
Masks are critical for the prevention of exposure to pathogenic airborne particulates. According to the emergency use authorization rules, N95 masks can be decontaminated up to three times using MSU’s VHP process. Sanitation extends mask life during shortages as well as saving health care dollars when PPE supply chains are stressed or interrupted.
“This is big: MSU experts working with Sparrow Hospital gave us the ability to sanitize masks for our health care workers fighting on the frontlines against COVID-19 –– and the FDA has approved that sanitization process," Slotkin said.
"Michigan, like so many other states, has been at the mercy of a medical supply chain with production, middlemen, and customs officials in other countries, and we just can’t let that happen again as we prepare for a potential second wave of COVID-19, said Slotkin, D-Holly. “Now that the FDA has approved this process, our community will be better prepared and protected if cases spike again. I’m grateful to the FDA for its approval and to the professionals at MSU for their hard work, dedication and ingenuity.”
Self-Adhesive N95 Mask has Several Unique Features
Global Safety First, LLC, (GSF) is a Pennsylvania limited liability company founded in early 2015 with the purpose of improving respiratory protection equipment for professionals and the general public. GSF develops, manufactures, and markets unique disposable safety masks and respirators (ReadiMasks), designed to substantially protect people from modern-day threats.
The ReadiMask was created to be virtually weightless, small enough to be carried at all times, and affordable for everyone. It is for use in (1) emergencies caused by accidents, terrorism, or natural disasters, (2) hospitals, medical offices, and other health care settings, (3) industrial settings, (4) the military, and (5) any environment where an individual is exposed, or may be exposed, to dangerous particulate contaminants.
The patented ReadiMask is a multipurpose respirator that substantially protects against airborne particles and is designed to fit both adults and children. It is the only full-face respirator that seals to your face using a hypoallergenic medical adhesive at the perimeter of the mask. Masks that apply with elastic straps or ties do not fit properly, allowing gaps around the edges of the mask where contaminants can enter, but ReadiMask’s medical adhesive creates a tight seal that enables the filter and eye shield to provide substantial protection. The ReadiMask is pocket-sized, virtually weightless, and available with or without the eye shield.

Outstanding Features:
- Hypoallergenic medical adhesive creates effective seal against airborne pathogens, and other harmful particulates
- Two-way protection (on inhalation and exhalation)
- Custom fit and available in three sizes
- High-tech filter media reduces breathing resistance
- High-quality audio communication
- Anti-fog eye shield with the option of wearing eyeglasses over the shield
- Portable and individually packaged for weightless protection on the go
- Latex-free
- Proudly made in the USA
Benefits:
- Unique airflow provides up to 10 degrees Fahrenheit cooler breathing air
- No painful elastic straps
- No painful metal nose clip
- Increased comfort and field of view
- Easy to fit test
- Flat configuration saves valuable space to support stockpiling
- Eyeglasses do not fog
Fauci: 'Disturbing Number' of COVID-19 Patients have Heart Inflammation
At a Senate hearing on COVID-19, Dr. Anthony Fauci on Wednesday addressed some lesser-publicized side effects seen among some COVID-19 "long-haulers": heart inflammation and cognitive abnormalities.
"A disturbing number of individuals" who have recovered from COVID-19 and "apparently are asymptomatic," "when they have sensitive imaging technologies such as magnetic resonance imaging or MRI," they're found to "have inflammation of the heart," Fauci said.
Fauci also said a symptom among COVID-19 "long-haulers" is "cognitive abnormalities," like the inability to concentrate.
"These are the kinds of things that tell us we must be humbled that we do not completely understand the nature of this illness,” stressed Fauci, director of the National Institute of Allergy and Infectious Diseases.
McIlvaine has calculated that the healthcare, economic and life quality costs of the average COVID case where there is recovery is $500,000. One of the variables is the longer term effects.
Genetic Changes in COVID Could Make Long Term Immunity Less Likely
Scientists in Houston on Wednesday released a study of more than 5,000 genetic sequences of the coronavirus, which reveals the virus’s continual accumulation of mutations, one of which may have made it more contagious.
That mutation is associated with a higher viral load among patients upon initial diagnosis, the researchers found.
The new report, however, did not find that these mutations have made the virus deadlier or changed clinical outcomes. All viruses accumulate genetic mutations, and most are insignificant, scientists say.
The new study, which has not been peer-reviewed, was posted Wednesday on the preprint server MedRxiv. It appears to be the largest single aggregation of genetic sequences of the virus in the United States thus far. A larger batch of sequences was published earlier this month by scientists in the United Kingdom, and, like the Houston study, concluded that a mutation that changes the structure of the “spike protein” on the surface of the virus may be driving the outsized spread of that particular strain.
Coronaviruses such as SARS-CoV-2 are relatively stable as viruses go, because they have a proofreading mechanism as they replicate. But every mutation is a roll of the dice, and with transmission so widespread in the United States — which continues to see tens of thousands of new, confirmed infections daily — the virus has had abundant opportunities to change, potentially with troublesome consequences, said study author James Musser of Houston Methodist Hospital.
We have given this virus a lot of chances,” Musser told The Washington Post. “There is a huge population size out there right now.”
Scientists from Weill Cornell Medicine, the University of Chicago, Argonne National Laboratory and the University of Texas at Austin also contributed to the study.
David Morens, a virologist at the National Institute of Allergy and Infectious Diseases, reviewed the new study and said the findings point to the strong possibility that the virus, as it has moved through the population, has become more transmissible, and that this “may have implications for our ability to control it.”
Morens noted that this is a single paper, and “you don’t want to over-interpret what this means.” But the virus, he said, could potentially be responding — through random mutations — to such interventions as mask-wearing and social distancing, Morens said Wednesday.
Wearing masks, washing our hands, all those things are barriers to transmissibility, or contagion, but as the virus becomes more contagious it statistically is better at getting around those barriers,” said Morens, senior adviser to Anthony S. Fauci, the director of NIAID.
This has implications for the formulation of vaccines, he said. As people gain immunity, either through infections or a vaccine, the virus could be under selective pressure to evade the human immune response.
“Although we don’t know yet, it is well within the realm of possibility that this coronavirus, when our population-level immunity gets high enough, this coronavirus will find a way to get around our immunity,” Morens said. “If that happened, we’d be in the same situation as with flu. We’ll have to chase the virus and, as it mutates, we’ll have to tinker with our vaccine.”
