
Coronavirus Technology Solutions
August 13, 2020
TSI Testing, Masks Ventilators and Isolation Room Air Pressure
Elmarco says High Efficiency Media Cost is Nominal
How Effective are the Aerosol Filtration Efficiencies for Fitted Face Mask Alternatives?
Oregon Schools Assessing HVAC Systems
Springfield Oregon Purchases 500 Air Purifiers
Large Hog Processor Shuts Down Due to COVID
Doctors Group Sues to Make Meat Processors Test Product for COVID
China Requiring Meat Processors to Certify that Products are Virus Free
Wisconsin Tribal Casino Installing UVDI
______________________________________________________________________________
TSI Testing, Masks Ventilators and Isolation Room Air Pressure
TSI Incorporated, designer and manufacturer of precision air particle measurement instruments, is working with healthcare organizations across the globe to enable the use of its technologies to ensure worker safety in the midst of the coronavirus, or COVID-19, pandemic. TSI's respirator fit testing, air flow measurement, mask filter testing and room pressure monitoring technologies are being utilized globally to help healthcare workers and first responders protect themselves as they care for affected patients.
"During this unprecedented global event, we are committed to doing our part to provide very specialized products and components that are critical to the protection and aid of healthcare workers, first responders, and of course, affected patients," said Tom Kennedy, Ph.D., President at TSI Incorporated. "This is personal for many of us at TSI as some of us have family members working on the frontlines of patient care or have at-risk family members. We are extremely proud of the role our people and products are playing during these difficult times, and our manufacturing and operations team deserve special recognition."
COVID-19 is a public health emergency and TSI recognizes that healthcare facilities and staff are on the frontlines. Given it is a respiratory illness, respiratory protection program (RPP) managers are tasked with finding solutions to better protect their colleagues. TSI recognizes this challenge and has been working closely with RPP teams to support training and implementation of its respiratory protection technologies, which also enable the collection of data to contribute to COVID-19 preparedness and infection prevention. Four ways the healthcare industry is currently utilizing TSI technology to help curtail the spread of COVID-19 include:
- Supporting protective mask manufacturers: Filter testers test filters, and filtration material and media. TSI designed and manufactures the 8130A Automated Filter Tester, which tests and certifies filter media and protective masks. As worldwide manufacturing capacity is ramped up, the capability of manufacturers to certify that the media and mask are fit for purpose and ensure proper filtration of harmful particles containing viruses is also increased. These devices are being utilized by facilities in the Wuhan, China area, where it was impossible to provide in person installation. TSI staff created virtual training and installation materials, enabling global partners to efficiently and effectively leverage the equipment.
- Supporting ventilator manufacturers: TSI manufacturers specially engineered an air flow sensor, providing highly accurate flow measurements required by ventilator manufacturers to ensure proper volumetric flow to patients in respiratory duress. In response to the unprecedented demand, TSI has brought additional fixturing and manufacturing equipment online and has ramped up production to meet the demand for this critical piece of medical equipment.
- Flattening the curve with respirator fit testing: Across the globe, TSI is rapidly delivering respirator fit testing equipment into the hands of agencies, healthcare workers and first responders. WHO, CDC, NIOSH and OSHA regulate N95 facemasks, which block at least 95% of airborne particles and are recommended for healthcare workers. Without a tight fit and annual fit test, N95 masks are not as effective. PortaCount® Respirator Fit Testers can fit test any respirator mask, including N95 masks, in two and a half minutes. Unlike qualitative tests – also known as "smell tests," a PortaCount® tester and its FitPro® software count the particles on both sides of a mask, providing quantitative results. Hospitals, clinics, fire and police stations, ambulance crews, military bases and nursing homes are choosing quantitative fit testing over the more subjective and time-consuming qualitative method.
- Supporting Hospital Isolation Rooms: TSI is a leading supplier of room pressure monitors and controls for Airborne Infection Isolation Rooms, helping keep patients and staff safe by ensuring that the room is properly pressurized to keep contaminants in, or out, depending on the patient situation.
Aerosol generators and dispersers play a vital role in producing the defined aerosol required for an array of aerosol testing, production, and research. Applications requiring aerosol generation are very diverse, and include, but are certainly not limited to, filter testing, deposition of nanoparticles onto surfaces, vacuum-cleaner performance tests, and calibration of aerosol instruments.
TSI, manufactures a full line of individual aerosol generators and dispersers capable of:
- Size: <10 nm - 200 µm
- Concentration: as high as 107 particles/cm3
- Composition: solid (dissolved or suspended), liquid or powdered
- Size distribution characteristics: monodisperse or polydisperse
Elmarco says High Efficiency Media Cost is Nominal
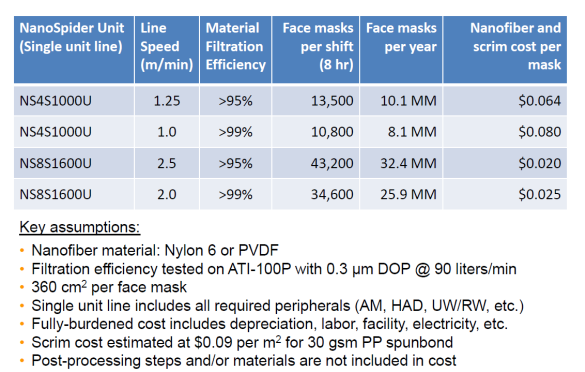
One of our topics for the webinar next week on masks will be the cost of higher efficiency. Elmarco can supply lines which can incorporate nanofiber media and scrim at only $0.020 per mask.
How Effective are the Aerosol Filtration Efficiencies for Fitted Face Mask Alternatives?
In this quality-improvement study of 29 fitted face mask alternatives, expired N95 respirators with intact elastic bands and masks that had been subjected to ethylene oxide and hydrogen peroxide sterilization had unchanged fitted filtration efficiencies (FFEs) of more than 95%, while the performance of N95 respirators in the wrong size resulted in decreased FFEs between 90% and 95%. As a group, surgical and procedure masks had lower FFEs relative to N95 respirators, with masks secured with elastic ear loops showing the lowest performance.
When new N95 respirators are unavailable, N95 respirators past their expiration date; sterilized, used N95 respirators; and other less common respirators can be acceptable alternatives.
Of the 29 different fitted face mask alternatives tested on 1 man and 1 woman, expired N95 respirators with intact elastic straps and respirators subjected to ethylene oxide and hydrogen peroxide sterilization had unchanged FFE (>95%). The performance of N95 respirators in the wrong size had slightly decreased performance (90%-95% FFE). All of the respirators not listed as approved in this evaluation (n = 6) failed to achieve 95% FFE. Neither of the two imported respirators authorized for use by the Centers for Disease Control and Prevention that were not NIOSH-approved tested in this study achieved 95% FFE, and the more effective of the two functioned at approximately 80% FFE. Surgical and procedural face masks had filtering performance that was lower relative to that of N95 respirators (98.5% overall FFE), with procedural face masks secured with elastic ear loops showing the lowest efficiency (38.1% overall FFE).
This quality-improvement study evaluating 29 face mask alternatives for use by clinicians interacting with patients during the COVID-19 pandemic found that expired N95 respirators and sterilized, used N95 respirators can be used when new N95 respirators are not available. Other alternatives may provide less effective filtration.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2769443
Oregon Schools Assessing HVAC Systems
Beaverton and Hillsboro School Districts say they are assessing schools to see which ones need upgrades.
"What it really comes down to is getting fresh outside air into our buildings and getting air circulating throughout the day," said Casey Waletich, chief operations officer for the Hillsboro School District.
At home, Waletich says people need to replace one air filter regularly. In a school district, he says districts need to replace hundreds.
"It’s quite an expensive endeavor for a district like ours. Looking at HVAC upgrades could be in the hundreds of thousands or millions of dollars depending on the significance of the upgrades we’d need," Waletich said.
Waletich says the district is in the process of assessing each school to determine what work needs to be done.
The Beaverton School District is also checking each school. The district says newer schools have state of the art HVAC systems. However, the district says the gym at West Tualatin View Elementary doesn't have an air filtration system.
State guidelines do say schools can open window or use fans to increase air flow.
Springfield Oregon Purchases 500 Air Purifiers
Springfield School District will install air purifiers in all of its schools in anticipation of students and employees returning to some form of in-person instruction this school year, when public health conditions allow.
The purifiers were just one aspect of reopening on the Monday night agenda for the Springfield School Board, along with a review of the district’s operational blueprint for fall, which is due to the state by next week.
Board members unanimously approved the purchase of the 500 new air purifiers at its meeting Monday night for a total of $265,000 in CARES Act money.
They’re called iWave air purifiers. The devices install into the schools’ HVAC systems and produce bipolar ions or positive and negative ions that purify the air as it comes through.
“When viruses or bacteria or mold come through, those ions disrupt the protein in those organisms and basically kill it,” said Terry Rutledge, assistant director for facilities and operations.
The purifiers also take pollen and air odors and turn them into different substances like water, oxygen or carbon monoxide, he said.
“So right now, yes, this is something that we take opportunity of because of COVID, and to make our buildings a lot safer and healthier for students and staff during this pandemic time, but as the end result when we’re done with the pandemic our schools are going to be healthier, regardless,” Rutledge said.
This is far from the only change being made in Springfield schools over the summer, although the purifiers play directly into plans for the coming school year, which was presented to the board.
iWave is an air purifying device that installs in any duct air conditioning system. When air passes over the iWave, ions produced by the device reduce pathogens, allergens, particles, smoke and odors in the air, creating a healthy environment without producing any harmful byproducts.
iWave uses patented technology, called needle-point bi-polar ionization, to create equal amounts of positive and negative ions. When these ions are injected into the air stream, they break down passing pollutants and gases into harmless compounds like oxygen, carbon dioxide, nitrogen and water vapor (see illustration below).
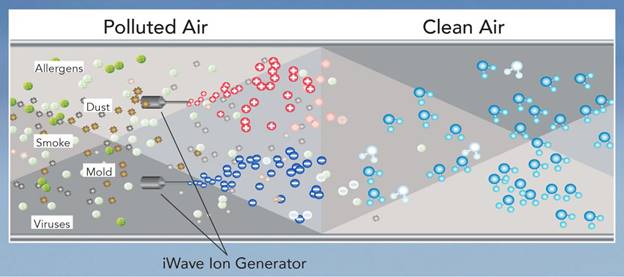
When the ions come in contact with viruses, bacteria or mold, they remove the hydrogen molecules – without them, the pathogens have no source of energy and will die. The ions also attach to allergens like pollen and other particles, causing them to band together until they are large enough to be caught by the ventilation system’s air filter.
iWave’s technology generates the same ions that nature creates with lightening, waterfalls, ocean waves, etc. Nature uses energy and shear to break apart molecules, naturally cleaning the air and producing a healthy environment. The only difference between the iWave’s technology and nature is that the iWave does it without developing harmful ozone according to the company.
Large Hog Processor Shuts Down Due to COVID
In an announcement that has shocked the industry, North Carolina-based Maxwell Foods (parent company Goldsboro Milling) has stated it will permanently close its hog production operations due to ‘projected financial losses’.
In a press release, Maxwell Foods stated that ‘the continuing low prices paid for our product, together with the effects of the Covid-19 pandemic, make the current and projected financial losses unsustainable for the company to continue operating’. Leaders at the North Carolina Pork Council added that ‘We are deeply saddened that the persistent and untenable economic conditions mean that Maxwell Foods has reached [this] difficult decision’. The 31 year-old firm will begin the shutdown this month with the aim of fully closing by mid-2021.
Doctors Group Sues to Make Meat Processors Test Product for COVID
The U.S. Agriculture Department acted unlawfully when it arbitrarily denied a petition to require meat and poultry processors to test products for the coronavirus and publish the results, the Physicians Committee for Responsible Medicine said in a complaint filed this month.
At least 40,517 meatpacking workers have tested positive for Covid-19 and about 189 have died, the group told the U.S. District Court for the District of Columbia. Studies have shown that the virus may be transmitted from these workers to the general public through infected meat and poultry, it said.
Yet, the agency won’t mandate testing or require processors to include information about the virus in their safe handling instructions, the group said. In July, it denied the group’s petition asking it to do so and to include a label stating that a product was processed in a facility where workers have tested positive for the virus.
USDA ignored evidence in the petition regarding conditions in processing plants that contribute to the high rate of infections among workers and baselessly concluded that the requested measures wouldn’t serve a public health purpose, the complaint said.
The group’s complaint also included claims that USDA hasn’t responded to Freedom of Information Act requests to identify the members of its Dietary Guidelines Advisory Committee and their ties to the food industry.
The committee is a nonprofit public health organization consisting of over 175 physicians and health-care professionals. It promotes proper nutrition as a means of preventive medicine, according to the complaint.
China Requiring Meat Processors to Certify that Products are Virus Free
China’s National Health Commission issued coronavirus control guidelines for meat processing companies, including demanding imported livestock and poultry products must be virus-free before processing in Chinese plants.
The guidelines come after a series of coronavirus outbreaks linked to meat processing plants across Europe and the Americas, with China banning imports from various origins.
Imported meats products must have certificates for passing nucleic acid tests for the coronavirus, on top of other documents, certificates and records of inspections, before being processed in the Chinese plants, the NHC said in a statement.
Meat processing enterprises should keep track of the source of poultry and meat, the NHC said, and establish a complete traceability mechanism.
Wisconsin Tribal Casino Installing UVDI
As tribal casinos reopen amid the ongoing pandemic, some are utilizing ultra-violet light systems in their ventilation ducts to help neutralize the coronavirus.
The Sokaogon Chippewa Community recently installed such a system made by the California-based company UltraViolet Devices, Inc., at its Mole Lake casino and lodge in Crandon.
“Tribal leadership is committed to enhanced measures to ensure the safety and well-being of our guests and employees as we reopen,” said Johnny L. Phillips Jr., assistant general manager of the Mole Lake Casino, in a statement. “As part of multiple facility upgrades, including touch-less restrooms, we’re pleased to work with UVDI to install its state-of-the-art UV air disinfection system.”
The tribe, which has around 1,400 members and a 4,900-acre reservation, has reported zero COVID-19 cases, which tribal officials attribute to enhanced testing and tracing.
The casino also has been closed for an extended period of time to limit potential coronavirus exposure, and tribal officials hope to keep COVID-19 cases at zero with safety protocols as they reopen, such as with UV light disinfectant.
“UV-C germicidal lamps are installed inside the HVAC system duct work,” said Will Gerard, spokesman for UVDI, about how the system works. “As the air passes the UV-C light, it ‘kills’ more than 99% of the virus immediately. The air is recirculated to the HVAC system from the casino space, therefore cleaning the air in the casino.”

The Forest County Potawatomi started working with UVDI in 2012 and again in 2017 and 2018 to install systems that would help eliminate odor from smoking at the tribe’s casino in Milwaukee.
Dave Brien, casino facilities director, said tribal officials also were aware that the system would help eliminate viruses when they were being installed.
“We’re filtering air to the level of a basic surgical suite,” he said. “Most systems only filter the outdoor air (coming in), but during a pandemic, it’s the indoor air you want to filter.”
The Milwaukee casino reopened June 8 with other safety measures still being practiced, such as checking the temperature of everyone entering the building, operating on limited hours and capacity, Plexiglas dividers installed between slot machines and increased sanitizing.
Brien said no one has contracted COVID-19 at the casino since reopening, to his knowledge.
He said that although the UV disinfection system is not advertised to customers, employees are aware it’s there, offering them more piece of mind.
Ultraviolet light technology was developed during the 1950s and is still in use in hospitals, but experts believe schools and other entities should consider installing the lights, which can cost $3,000 per classroom.
“The pandemic has driven inflection point growth in demand for our UV-C air and surface disinfection products,” Gerard said.
The company has been in business more than 70 years and its systems are installed in more than 10,000 buildings globally, including hospitals and museums.
Brien said installation of the UVDI system wasn’t cheap, but “it was the right thing to do.”
“We are always looking at what we can do to improve the air quality in our casinos,” he said.
Brien said tribal officials are researching needlepoint plasma technology that’s now available that would also eliminate the virus without the use of UV light.
