
Coronavirus Technology Solutions
June 24, 2020
Treat Each Person as a Cleanroom
Product
Radiant Fibertech PVT Supplier
of Face Mask Fabric
Suominen Introduces New Material
for EN Type II Facemasks
AKAS Layered Fabrics Outperform
N95 Meltblown in Filtration
Efficiency
Jacob Holm Teams with Under
Armour on Face Masks
H&V Continues to Expand Capacity
to Mitigate
COVID
Nanofiber Masks Compare
Favorably in Both Efficiency and
Comfort
Investors Discuss New Startups
in the COVID Battle
____________________________________________________________________________
Treat Each Person as a Cleanroom
Product
To protect pharmaceutical
products from airborne
contamination we do not consult
with doctors or epidemiologists,
we instead rely on cleanroom
specialists.
We do not rely on distancing the
product from the source of
contamination instead we protect
the product by assuring that the
air with which it comes in
contact is as free of
contaminants as possible.
Billions of dollars have
been spent by the cleanroom
industry to achieve this goal.
It is their expertise
which needs to be absorbed by
government decision makers.
COVID is not transmitted by
fleas, mosquitoes or
contaminated food. Transmission
through touching surfaces is now
being downgraded as a source.
The consensus main source is
aerosols.
There is disagreement
about what percentage is from
small aerosols which travel long
distance as
opposed to those that
travel less than six feet.
But in either case the
purification of the air being
inhaled is the solution.
We are relying primarily on the
advice of physicians and
epidemiologists in making what
has proven to be some disastrous
decisions (e.g. not wearing
masks and not worrying about
HVAC systems).
These would be the first
areas of focus for a
cleanroom designer. The new
evidence of the way COVID is
transmitted should lead us to
cleanroom experts and treat
people the way we would a
valuable pharmaceutical product.
Radiant Fibertech PVT Supplier
of Face Mask Fabric
Radiant Fibertech Pvt. Ltd.
started the production of
nonwoven fabric in 2012 with the
brand name REDIFIL. The Indian
manufacturing plant is located
on Rajkot – Gondal National
Highway No. 8-B, at Bhunava. The
prime aim for selecting the
plant location is the proximity
of the plant to transportation
facility, continuous power
supply and availability of
skilled & semiskilled labors.
With the main objective of
protecting the environment from
degradation, they are committed
to supply quality nonwoven
fabric made from 100%
Polypropylene which is durable,
attractive, cost effective and
eco-friendly. It is producing
nonwoven fabric from 10 GSM to
200 GSM with maximum width of
1600 mm with more than 25
attractive colors. The installed
capacity of the
plant is 1800 mt./annum
which is used for
·
Surgical Gowns & Aprons
·
Face masks,
·
Gloves & caps
·
Top sheets of Baby Diapers
·
Fastener tapes for Baby
Diaper[BM1]
Suominen Introduces New Material
for EN Type II Facemasks
Suominen has developed a
nonwoven material for the
manufacture of facemask
applications. The new nonwoven
has passed European Standard EN
14683:2019 Type II requirements
in terms of filtration
efficiency and pressure drop.
“Our FIBRELLA Shield nonwoven
has excellent filtration
efficiency and pressure drop
values meaning that the material
provides protection while being
comfortable and easy to breathe
through. Measured with an
applied method by VTT results
indicate that FIBRELLA Shield
nonwoven’s filtration efficiency
is higher than 99% reaching type
II requirements but of course
the material can also be used
for lighter model Type I masks
or uncertified masks,” said
Category Manager, Johanna Sirén.
The standard EN14683:2019 for
medical masks is for end
products and the converter has
to repeat the tests to confirm
the standard compliancy for the
end product. The end product
needs to comply also with the
regional regulations, if any.
Developed in cooperation with
VTT, this new material is the
latest addition to the FIBRELLA
family. FIBRELLA Shield is
already in production at
Suominen’s Nakkila plant in
Finland. Currently the plant is
capable of producing material
for approximately 15 million
masks per month.
AKAS Layered Fabrics Outperform
N95 Meltblown in Filtration
Efficiency
A new study by Northeastern
University found that a facemask
constructed using fabrics
manufactured by AKAS Textiles, a
Pennsylvania-based textile
manufacturer, outperformed an
N95 respirator in an aqueous
media under positive pressure of
20 Kilo Pascal, simulating a
sneeze/cough. The study tested
more than 70 different common
fabric combinations and masks,
including the N95 respirator,
for their ability to block the
transmission of virus-like
nanoparticles. The mask with the
best filtration was made of
layers of ProCool Performance
Fabrics combined with Zorb 3D
Stay Dry Dimple fabric. The
combination of these fabrics
tested 72% more effective than
the N95 respirator.
The study was published in ACS
Nano, a monthly,
peer-reviewed scientific journal
published by the American
Chemical Society. The authors
wrote, “Layered systems of
commonly available fabric
materials can be used by the
public and healthcare providers
in face masks to reduce the risk
of inhaling viruses with
protection that is about
equivalent to or better than the
filtration and adsorption
offered by 5-layer N95
respirators. The masks were
evaluated with steady-state,
forced convection air flux with
pulsed aerosols that simulate
forceful respiration”.
Jacob Holm Teams with Under
Armour on Face Masks
Jacob Holm, the manufacturer of
Sontara fabrics and other
nonwovens for medical and wipes
applications, is ramping up
material for PPE. The company
will implement a company-wide
investment program that would
lead to a 500 million square
meter capacity expansion
annually. The program, called
Project Boost, began last month
and will be completed by the
third quarter of 2022.
“Project Boost is our response
to the needs of our partners
across the globe for increasing
capacity, providing more
sustainable substrate choices
and continuing to hold our
position as an innovation leader
in nonwovens,” says CEO Martin
Mikkelsen.
Last month, the company reported
that it had seen a 65% increase
in its Sotnara medical fabric
during the Coronavirus pandemic,
leading two of its five sites to
report record production levels
in April.
“This situation is
unprecedented,” Mikkelsen says.
“However, for Jacob Holm as a
company, the only way forward is
to lean in to what we know and
use the full force of our
experience to help contain the
spread of COVID-19 and make a
positive impact on the wellbeing
of our communities.” Among Jacob
Holm’s efforts in the fight
against Covid-19 was a
partnership with sport
performance brand Under Armour
to make face masks and isolation
gowns.
H&V Continues to Expand Capacity
to Mitigate
COVID
Hollingsworth & Vose, has
shifted its focus beyond its
traditional markets like
medical, automotive and
filtration to create a material
for non-surgical gowns on an
accelerated timeline. “H&V had
already been manufacturing
critical materials used in the
fight against Covid-19 including
filtration media for N95
respirators, ventilator
filtration media and the
materials used in surgical hoods
so it made sense for us to see
how else we might be able to
meet the needs of healthcare
workers on the front lines,”
says Jeff Crane, segment leader,
H&V.
Nanofiber Masks Compare
Favorably in Both Efficiency and
Comfort
A research team led by Professor
Ick Soo Kim of Shinshu
University's Institute for Fiber
Engineering (IFES) looked into
the effectiveness of sterilizing
N95 masks. They looked at
commercially available
melt-blown nonwoven fabric N95
masks and nonwoven nanofiber
masks with N95 filters. They
examined the filtration
efficiency, comfort of the
wearer, and filter shape change
after washing and disinfecting.
The methods of disinfection test
involved directly spraying 75%
ethanol on the mask filter and
air drying and soaking the mask
filter in 75% ethanol solution
for 5 minutes to 24 hours and
leaving it to air dry naturally.
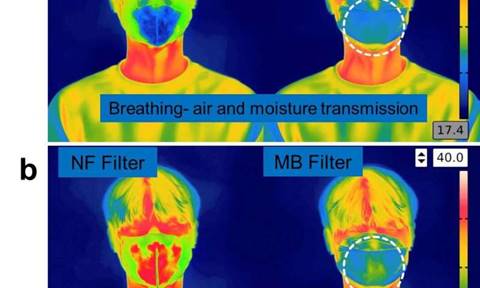
Filtration efficiency of both of
the filters (melt-blown filter
and the nanofiber filter) was
95% or more before use, which
indicates that the respiratory
organs of the wearer can be
effectively protected. The tests
also clarified that the inside
of the filter can be effectively
sterilized by spraying ethanol
three times or more or immersing
it in an ethanol solution for
more than five minutes. However,
when the mask was reused after
the ethanol disinfection, the
filtration efficiency of the
melt-blown filter decreased to
64%. On the other hand, the
nanofiber filter did not
deteriorate in filter
performance even after ten or
more uses.
Melt-blown filters work on the
principle of electrostatic
charge for the removal of
particulate matter, as in the
result of ethanol spraying or
dipping the electrostatic charge
on the surface of melt-blown
filter was lost, so efficiency
of melt-blown filter was
significantly decreased. On the
other hand, filtration mechanism
of nanofiber filter is
independent of static charge and
fully dependent on pore
diameter, pore distribution, and
morphology of nanofibers. As in
the result of disinfection,
morphology of nanofibers was not
affected, thus it also
maintained its filtration as
optimum as it was before use.
In addition, the nanofiber
filter has higher heat emission
and carbon dioxide emission
performance than the melt-blown
filter and exhibits excellent
breathability. Similarly, it was
confirmed that the nanofiber
filter had lower cytotoxicity
than the melt-blown filter when
a safety experiment using human
skin and vascular cells was
performed.
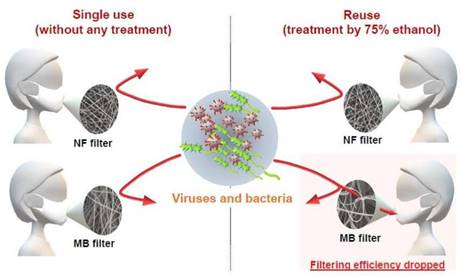
Comparison of mask filter
performance after ethanol
disinfection: The nanofiber
filter has air permeability even
after being washed. The
melt-blown filter has its mesh
structure changed by ethanol
sterilization and its
performance is deteriorated.
As stated above, both mask
filters have similar filtering
performance at the time of first
use, but after disinfecting and
reusing, the nanofiber filter
does not exhibit performance
deterioration. In other words,
nanofiber filters can be easily
sterilized with ethanol at home
and reused multiple times.
"This research is an
experimental verification of the
biological safety of nanofiber
masks and the maintenance of
filtration efficiency after
washing, which has recently
become a problem," Professor Cha
Hyung Joon states, who
co-presided the research.
Professor Ick Soo Kim hopes
that nanofiber masks will serve
as a means of prevention in the
second and third wave of
coronavirus infections.
Investors Discuss New Startups
in the COVID Battle
This is a summary of a live
panel ran by SOSV to
introduce and discuss solutions
funded by some of the most
active investors in the field.
Each of the three funds (Fifty
Years, Khosla
Ventures, SOSV)
had published an impressive list
of their relevant portfolio
startups. IndieBio even made a call
to fund Covid-fighting startups as
part of its newly launched NYC
program.
Protecting our face
-
Many masks don’t
effectively
protect against
infection as the
virus is too
small (about
120nm). Verdex (a
SOSV portfolio
company) has
developed a
nanofiber
material that
filters out
particles above
100nm —
effectively
blocking the
virus — and is
also more
breathable.
-
HabitAware,
a HAX portfolio
company, had
created a
machine-learning-powered
bracelet to
prevent
body-focused
repetitive
behaviors (e.g.
nail biting) by
recognizing and
alerting of
specific
gestures. The
pandemic
provided a new
direct
application of
their technology.
Disinfecting everything
The ‘new normal’ is making
frequent and thorough
disinfection of our living and
working environment necessary,
to protect us and helps us get
back to work.
-
HAX has invested
in various
solutions for
this, from floor
cleaning (Avidbots)
to toilet
cleaning (Somatic).
-
The dry cleaning
robot startup
Presso announced
its solution initially
intended for
business travels
was now in
high-demand with
movie and TV
studios who are
resuming
operations.
-
Youibot,
an autonomous
logistics
startup, managed
to repurpose
their technology
to provide
disinfection
with UV-C
lights, and
temperature
detection.
Testing with devices
On the testing front, several
countries have introduced
testing stations that look like
phone booths. Some are also
using helmets with IR sensors to
detect potential infections.
Some wearable device companies
like Oura and Strados
Labs are
applying their technology to
pre-symptom detection or
monitoring.
Treatment
Treating the virus is
complicated, as it might also
involve treating the immune
response, and support the
recovery of patients.
·
Various large and small
companies are working on vaccine
candidates,
·
Some companies are trying to
develop new drugs, or repurpose
existing ones for a faster
time-to-market
The lack of equipment —
particularly respirators — is
being addressed partly by
repurposing other devices. Among
them are snorkeling masks and
BIPAP/CPAP machines. Decathlon
donated their stock of
snorkeling mask to hospitals.
Finally, alongside this pandemic
comes tremendous emotional harm
due to stress, economic
uncertainty and unemployment.
Some companies such as Feel are
working on low-cost solutions to
help people improve their mental
health.
Seth Bannon, Founding Partner at
Fifty Years
-
The Covid-19
threat is
reminiscent
of WWII, with
potential deaths
in the tens of
millions.
-
WWII gave us
many tech
innovations such
as: mass
production of
antibiotics,
blood plasma as
a therapeutic
solution, skin
grafts, flu
vaccine, radar,
microwave ovens,
pressurized
plane cabins,
nuclear power
and the first
programmable
digital
computer. It
laid the
foundation of
technical
progress for
many years to
come. A similar
rally today
could build
solutions for
the future.
-
Everyone who can
help should
help, and 17
of our portfolio
companies did.
-
HelixNano (from
the George
Church lab at
Harvard).
Working on
a vaccine to
counteract
SARS-Cov-2
evolutions and
antigenic drift.
-
BillionToOne found
a way to run
a test on Sanger
sequencers at
low cost and
high volume.
-
Opentrons (a
co-investment
with Khosla and
SOSV) built a
low-cost lab
robot to
automate liquid
handling,
already deployed
in multiple labs
around the world
to test covid.

An Opentrons robot in Italy
(source: 24ORE)
-
Voodoo
Manufacturing directed
their cloud farm
of 3D printers
to focus on combating
Covid-19 by
producing PPE
and more at
cost.
-
Solugen,
that makes
hydrogen
peroxide,
realized they
had the capacity
to
make hand sanitizer and
now do so
pro-bono.
Alex Morgan, Partner at Khosla
Ventures
-
Khosla has over
$1B under
management,
including a main
and seed fund.
-
The goal is to
‘reinvent social
infrastructure
with
technology’,
looking for
investments
combining
financial
returns with
societal impact
(e.g. Impossible
Foods, Color,
etc.). We
also have a list
of companies
responding to
Covid-19.
-
Genalyte has
a
FDA-approved testing solution that
takes 15min.
Current capacity
is about~250k
patients /
month.
-
Luminostics designed
an optical test that
can be run with
a small device
attached to a
phone.
-
Pardes Bio is a
recent
investment
working on
a therapeutic using
a protease
inhibitor.
-
Prellis
Biologics (IndieBio/SOSV
co-investment)
is a tissue
engineering
company that can
3d print lymph
nodes ex vivo to
produce therapeutic antibodies (here
are a recent video
interview and media
coverage).
-
Some other
investments also
focus on
the distribution
of care, and
particularly mental
health, such as Ginger (remote
service for
mental health)
and Flow
Neuroscience (a
SOSV/HAX
co-investment
offering a
drug-free
treatment for
depression using
an at-home brain
stimulation
device, already
on sale in
EU/UK).
Jun Axup, Chief Science Officer
& Partner at IndieBio
-
IndieBio is
the life
sciences
accelerator
program of SOSV,
based in SF and
NYC and
investing
globally. It
invested in 136
companies
including
Memphis Meats,
Clara Food and
Perfect Day in
the cellAg
space.
-
CASPR Biotech uses
·
CRISPR for low-cost testing
(covered by NYT).
·
Renegade.Bio was
founded as a lab to test at high
speed and large volume.
·
ANA
Therapeutics is
repurposing an anti-worm
treatment toward Zika, SARS and
now Covid.
·
Halomine found
a way to keep surfaces free of
viruses by stabilizing chlorine,
making the protective film last
up to 30 days instead mere
hours.
