
Coronavirus Technology Solutions
June 3, 2020
The World is One Large Coronavirus Cleanroom
Market
How Long Does the COVID Remain a Threat on
Surfaces?
Study on Hamsters Confirms Efficacy of Face
Masks
Contec Expands Supply of Sporicidin Disinfectant
Silicon Based Membrane for Masks has Efficiency
and Other Advantages
Black & Veatch Develops Boarding Pass to Screen
Employees for COVID Aspects
Short-waved UVC Irradiation is Highly Effective
for Inactivating SARS-CoV-2 Viruses
Antimicrobial Door Handle Cover Available from
Teknomek
Cherwell Laboratories Optimistic About Viable
Air Monitors
Redditch Medical
has New Hand Hygiene Product
Sweden With No Lockdown Has Had Too Many Deaths
230,000 Healthcare Workers have Contracted COVID
Fibre Extrusion Technology Says There May Be
Better Answers Than Polypropylene for Meltblowns
______________________________________________________________________________
The World is One Large Coronavirus Cleanroom
Market
We live in a world where the coronavirus plays a
major role now and for the foreseeable future.
What we are finding is that this virus
can travel in small droplets released by the
lungs or on small particles of which there is no
shortage. We breath in and exhale millions of
tiny particles every hour. With this new finding
we need to turn to the cleanroom experts who
have been focused on eliminating small particles
from the air for 60 years.
These experts and the products and services they
offer provide an essential resource for
mitigating the virus. McIlvaine is assisting by
providing the suppliers with forecasts of the
market opportunities in the broader vision of
the world as one big coronavirus clean room
market.
World Cleanroom Markets
forecasts the revenues of rooms, components,
masks and other consumables for all industries
including biopharmaceuticals and hospitals.
http://home.mcilvainecompany.com/index.php/markets/other/n6f-world-cleanroom-markets
Cleanroom Technology Solutions
with daily alerts and webinars analyzes the
masks, filters, and various other hardware and
consumables which will allow a safe return to
near normal life and work.
http://home.mcilvainecompany.com/index.php/markets/air/82ai-coronavirus-market-intelligence
Coronavirus Pharmaceutical Solutions
tracks the progress to develop vaccines,
therapeutics, and diagnostics.
Bi-weekly Alerts are accompanied by
detailed profiles of the developers and contract
manufacturers who make mitigation possible.
June 3 Alert, Gilead
All three of these services are being offered as
a package and included with the World
Cleanroom Markets report at no extra charge.
A supplier needs all three of these services to
fully understand the opportunities and
challenges created by COVID.
A cleanroom is defined in the ISO standard
14644-1 as:
“A room in which the concentration of airborne
particles is controlled, and which is
constructed and used in a manner to minimize the
introduction, generation, and retention of
particles inside the room and in which other
relevant parameters, e.g. temperature, humidity,
and pressure, are controlled as necessary”
6.2 million people have contracted COVID 19 in
the last six months.
380,000 have died. With a problem of this
magnitude caused in large part by an airborne
virus we have to be thinking of the world as one
big facility which needs to adopt cleanroom
technology. The same strategy can be applied as
would be applied to a pharmaceutical complex.
In a pharmaceutical facility there is likely to
be a progression of clean spaces.
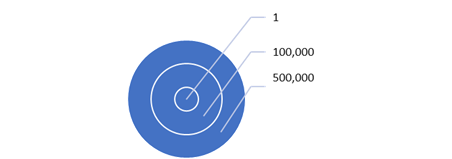
Ambient air in a typical city contains 500,000
or less particles 0.3 microns in diameter in
each cubic foot. It also contains millions of
smaller particles. An individual inhales the air
with the particles and then exhales the CO2 and
sends the particles back into the environment. A
super spreader can be generating thousands of
virus aerosols per minute. Some will be in small
droplets caused by lung splashes. They
may
travel hundreds of yards. Those which attach to
small particles can drift over a whole region
e.g. Lombardy, Italy.
There is no knowledge as to what
percentage is inactivated by distance traveled
and what percentage is just dormant and ready to
be revived in lung moisture.
There is research which indicates that the
disease can be transmitted by as few as 10 virus
particles. On the other hand experts say that
most is contracted through contact with large
numbers of particles. As long as some of the
COVID transmission is through aerosols there
will be no fool proof way to prevent COVID
transmission. A mitigation program has to
therefore accept some risk and minimize the
transmission as much as is economically
possible.
This is the same strategy used in a
pharmaceutical facility. Many pharmaceutical
operations take place in space where the number
of 0.3 micron particles is limited to 100 or
less. Within that space there may be isolators
where there is less than one 0.3 micron particle
per cubic foot. When one leaves a less clean
space and enters a cleaner area there are
possibly air showers, garment changing and other
procedures.
The decision on how clean to make each space is
a function of risk and cost. The same principle
applies to dealing with COVID. The protection
effort needed on a crowded subway is much
greater than a sparsely inhabited park.
Suppliers have products to address varying
levels of risk and reduction.
An advantage of the packaging of the three
services is to determine the impact of one on
the others. The massive effort to create
vaccines, therapeutics, and diagnostics for
COVID means that there will be reduced cleanroom
revenues for cancer and other
biopharmaceuticals.
For more details on this package contact Bob
McIlvaine at 847 226 2391 or email him at 847
226 2391.
How Long Does the COVID Remain a Threat on
Surfaces?
CWS has posted information on coronavirus
tenacity at
https://www.cws.com/en-IE/news/how-long-does-coronavirus-survive-surfaces-2020-05-28.
Currently, nobody knows exactly how long
SARS CoV-2 - as the virus is correctly named -
"survives" on surfaces or, better, how long SARS
CoV-2 remains infectious on a surface. This
depends on its tenacity. This refers to
biological toughness or resistance to
environmental influences.
However, the question of how long the virus
remains active is very important, especially at
this time, and in the cleanroom. It can only be
answered by conducting experiments. There could
be the following possibilities:
-
"Genetic" detection: Comparable
to the corona test on patients,
a sample ("smear") from the
surface is used to measure how
much of the virus’ genetic
information, known as RNA, can
be detected. This allows us to
know whether the virus is or was
present. However, the result
does not tell us anything about
whether the virus is actually
still infectious.
-
Biological cell detection: The
sample, including the virus, is
added to a cell culture. The
virus infects the cells, causing
them to change or die. These
changes can be viewed with a
microscope. If different
dilutions – known as titre
levels - of the virus sample are
added to the cells, quantitative
information on the infectious
potential is obtained. If the
surface is examined at different
time intervals, information can
be obtained on how long the
virus can remain infectious.
In fact, there is little data on the tenacity of
SARS CoV-2. For the closely related
coronaviruses SARS CoV-1 (causative agent of the
pandemic 2002/03) and MERS (Middle East
Respiratory Syndrome, causative agent of the
epidemic on the Arabian Peninsula in 2012), a
tenacity of up to 120 hours could be
demonstrated in experiments.
The data for the various coronaviruses differ
greatly. With regard to the environmental
conditions, however, it is clear for all
viruses: the tenacity decreases
-
with increasing ambient
temperature
-
with rising humidity
-
with low "load", i.e. number of
viruses/unit area
The table summarizes the "survival times", i.e.
the tenacity determined for different surfaces.
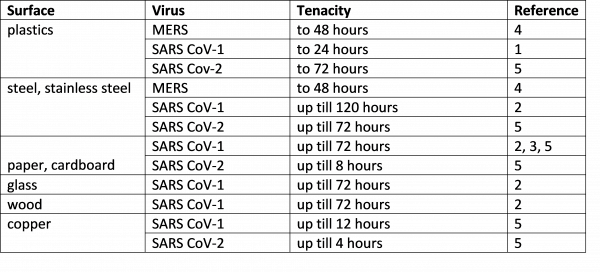
References: 1) CHAN et al. 2011; 2) DUAN et al.
2003; 3) LAI et al. 2003; 4) VAN DOREMALEN et
al. 2013; 5) VAN DOREMALEN et al. 2020
In summary, this means that:
The data unanimously show a high tenacity for
the SARS CoV-2 virus, particularly on plastic
and stainless steel surfaces. In principle,
effective barriers already exist against the
penetration of viruses into cleanrooms. However,
the high "survival times" of SARS CoV 2 demand
and justify further measures for surface
disinfection in the cleanroom itself as well as
in the access area, so that the distribution of
the virus across contaminated surfaces can be
limited. This applies in particular to surfaces
that are frequently contacted, such as door
handles, switches, door surfaces or seating.
Since CoV-2 is an enveloped virus, "limited
virucidal" disinfectants are suitable for
additional disinfection measures. These are
based on ethyl alcohol and/or isopropyl alcohol,
are easy to use and reliably inactivate SARS
CoV-2.
The source of this information was profi-con
GmbH and reinraum-akademie
info@reinraum-akademie.de.
Study on Hamsters Confirms Efficacy of Face
Masks
A study by University of Hongkong researchers
demonstrated that airborne transmission was a
major mode of transmission and that facemasks
were efficient in minimizing the transmission
The researchers
used a
well-established golden Syrian hamster
SARS-CoV-2 model. They
placed
SARS-CoV-2-challenged index hamsters and naïve
hamsters into closed system units each
comprising two different cages separated by a
polyvinyl chloride air porous partition with
unidirectional airflow within the isolator. The
effect of a surgical mask partition placed in
between the cages was investigated. Besides
clinical scoring, hamster specimens were tested
for viral load, histopathology, and viral
nucleocapsid antigen expression.
Non-contact transmission was found in 66.7%
(10/15) of exposed naïve hamsters. Surgical mask
partition for challenged index or naïve hamsters
significantly reduced transmission to 25% (6/24,
P=0.018). Surgical mask partition for
challenged index hamsters significantly reduced
transmission to only 16.7% (2/12,
P=0.019) of exposed naïve hamsters.
Unlike the severe COVID-19 manifestations of
challenged hamsters, infected naïve hamsters had
lower clinical scores, milder histopathological
changes, and lower viral nucleocapsid antigen
expression in respiratory tract tissues.
The conclusion was that SARS-CoV-2 could be
transmitted by respiratory droplets or airborne
droplet nuclei in the hamster model. Such
transmission could be reduced by surgical mask
usage, especially when masks were worn by
infected individuals.
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa644/5848814?searchresult=1
Contec Expands Supply of Sporicidin Disinfectant
The demand for antibacterial cleaning supplies
continues to climb in response to the COVID-19
pandemic, and healthcare institutions are
looking for effective alternatives to
traditional solutions. To fill this need, Contec,
the critical cleaning products and cleanroom
supplies provider, is scaling up production of
its Sporicidin brand disinfectant, most often
used for mold and water damage remediation, and
turned to diversified global manufacturer and
materials science expert Milliken & Company to
help produce mass quantities.
Since 1978, Sporicidin brand disinfectant
products have been used for infection and
contamination control by hospitals, medical and
dental offices, veterinary clinics, and
restoration professionals.
The EPA-registered intermediate-level
disinfectant cleans, disinfects and deodorizes,
and it provides 100% kill of pathogenic
vegetative organisms, including MRSA, VRE and
Avian Influenza A Virus (H9N2 and H1N1) with
continuous residual activity for up to six
months.
Compatible with plastics, wood, glass and
metals, alcohol-free Sporicidin brand
disinfectant is non-staining, non-abrasive and
non-corrosive. Notably, the disinfectant carries
a Category IV EPA toxicity rating the lowest
toxicity rating given to antimicrobials.
"Milliken immediately came to mind when we
decided to bring on a new manufacturing
partner," said Jack McBride, Contec CEO.
"Milliken is a trusted, key community player
with the mass production, quality systems and
speed-to-market capabilities needed to help us
provide critical cleaning solutions to
healthcare facilities without delay."
Sporicidin production at Milliken began on
Tuesday, 12 May, after a record ramp-up of only
four weeks. Typical similar partnerships require
three to six months to arrange.
"Companies are adapting their core competencies
to meet the global challenges brought on by
COVID-19," said Halsey Cook, President and CEO
of Milliken. "Partnering with Contec was a
natural fit and gave Milliken the opportunity to
quickly reconfigure our manufacturing
capabilities and rapidly solve problems for our
customers and communities."
Milliken quickly undertook an intensive
technical process to manufacture Sporicidin
brand disinfectant on behalf of Contec, Inc.
Enabled by its research, development and
manufacturing expertise, Milliken completed all
EPA requirements and implemented training and
protocols to safely produce a quality
disinfectant.
Sporicidin brand disinfectant is available in
ready-to-use 32 oz (0.65L) spray bottles,
1-gallon (3.8L) containers and pre-saturated
wipes.
Silicon Based Membrane for Masks has Efficiency
and Other Advantages
Researchers funded by King Abdullah University
of Science and Technology
have developed a membrane that can be attached
to a regular N95 mask and replaced when needed.
The filter has a smaller pore size than normal
N95 masks, potentially blocking more virus
particles.
N95 masks filter about 85% of particles smaller
than 300 nm, according to published research.
SARS-CoV-2 (the coronavirus that causes
COVID-19) is in the size range of 65–125 nm, so
some virus particles could slip through these
coverings. Also, because of shortages, many
health care workers have had to wear the same
N95 mask repeatedly, even though they are
intended for a single use. To help overcome
these problems, Muhammad Mustafa Hussain and
colleagues wanted to develop a membrane that
more efficiently filters particles the size of
SARS-CoV-2 and could be replaced on an N95 mask
after every use.
To make the membrane, the researchers first
developed a silicon-based, porous template using
lithography and chemical etching. They placed
the template over a polyimide film and used a
process called reactive ion etching to make
pores in the membrane, with sizes ranging from
5–55 nm. Then, they peeled off the membrane,
which could be attached to an N95 mask. To
ensure that the nanoporous membrane was
breathable, the researchers measured the airflow
rate through the pores. They found that for
pores tinier than 60 nm (in other words, smaller
than SARS-CoV-2), the pores needed to be placed
a maximum of 330 nm from each other to achieve
good breathability. The hydrophobic membrane
also cleans itself because droplets slide off
it, preventing the pores from getting clogged
with viruses and other particles.
https://pubs.acs.org/doi/full/10.1021/acsnano.0c03976
Black & Veatch Develops Boarding Pass to Screen
Employees for COVID Aspects
As return-to-work programs kick off across the
United States, Black & Veatch announced the
launch of COVOPERATE, an innovative new
workforce management application powered by
Field2Base that can help employers navigate the
complex challenge of safely and productively
restoring operations impacted by the COVID-19
pandemic.
Part of a comprehensive safety suite augmented
by on-site, physical screening resources,
COVOPERATE is a flexible, scalable cloud-based
application that tracks and manages
professionals’ critical health data. COVOPERATE
relies on a series of screening questions and
health declarations paired with in-person
temperature checks and other safety protocols to
generate an easily trackable “boarding pass” to
assist in evaluating if professionals are clear
to return to work or require additional
verification.
“Black & Veatch worked closely with Field2Base
to design an easy to use ‘boarding pass’ type of
solution for businesses, schools and commercial
groups looking to minimize risk in their return
to work programs,” said John Chevrette,
president of Black & Veatch Management
Consulting. “By collecting specific health data
determined by each organization, COVOPERATE
quickly evaluates an individual’s return to work
status and shares it with on-site resources that
can determine appropriate site access.”
By digitizing information and securely managing
the data in a secure, HIPAA-compliant and
audited platform hosted by Field2Base,
COVOPERATE helps organizations ranging from
commercial and industrial businesses to
education, government and financial organization
settings to assist in establishing enhanced
safety site access programs. In addition, the
data provided by COVOPERATE can also assist in
management of complex, daily labor workflows by
providing location validation and a real-time
view into available staffing resources.
Based in North Carolina, Field2Base is a
software company that specializes in developing
field service automation and mobile form
solutions. The company will manage the
collection and processing of professionals’
health data and provide technical support for
COVOPERATE at the organization level.
The application is currently in market testing
with select large enterprise companies across
the U.S. and will support access management for
millions of workers. Black & Veatch will also
begin to use COVOPERATE to help foster the
long-term safe return of its professionals.
“We are thrilled to work with Black & Veatch and
to rely on their engineering leadership and
combine our mobile technology expertise to
design COVOPERATE,” said Ed White, CEO and
Chairman, Field2Base. “Helping organizations get
back to work safely and productively will be
critical as we continue to navigate the global
impacts of COVID-19.”
A global leader in critical infrastructure
services, Black & Veatch has introduced several
solutions that support business activities and
return-to-work efforts impacted by COVID-19,
including BV Safe Contact, a
cloud-hosted geospatial tracking tool designed
to help keep field services and construction
crews working on critical infrastructure
projects safe, and the Rapid Modular Health
System (RaMHS), which provides testing and
screening capabilities outside of traditional
healthcare or business security settings.
Short-waved UVC Irradiation is Highly Effective
for Inactivating SARS-CoV-2 Viruses
UV specialist company Dr Hönle has shown that
energy-rich short-waved
UVC irradiation is highly effective for
inactivating SARS-CoV-2 viruses. The tests
were conducted at the Institute for Medical
Virology of the University Hospital Frankfurt.
The results showed coronavirus can be killed
reliably within seconds using Hönle UV units. An
inactivation rate of 99.99% (log4) was confirmed
in the laboratory.
The tests were carried out applying different UV
technologies and showed a clear result: Whether
the disinfection units were equipped with UVC
discharge lamps or UV-LED, inactivation rate and
inactivation time remained constant and
reproducible.
The results concluded that the risk of infection
with COVID-19 is reliably and efficiently
minimized by disinfecting ambient air and
surfaces with UVC irradiation. Hönle used these
results for their latest product series
STERICUBE and STERIAIR, consisting of UVC
cabinets, UVC chambers and UVC hand lamps for
germ inactivation.
Antimicrobial Door Handle Cover Available from
Teknomek
Hygienic furniture specialist Teknomek has
launched easy fit antimicrobial door handle
covers to help businesses manage transference
risks throughout a pharma facility. The product
helps prevent cross-contamination from one user
to the next in under 30 seconds.
Sue Springett, Commercial Manager at Teknomek,
said: "Our sanitizing door handles that release
viricidal gel have unsurprisingly been in great
demand over the course of the COVID-19 pandemic.
While it makes sense to install these at high
risk areas such as the entrance to a cleanroom
or washrooms, the need for antimicrobial
management extends beyond these key areas."

"The new antimicrobial door handle covers are a
quick fit solution adding an additional layer of
protection and reassurance to staff," Springett
added. "Most importantly, they help reinforce
the importance of hygiene culture from the
moment a person arrives at the building, at a
time when this has never been more important."
The hygienic handle covers feature a silver ion
antibacterial coating that kills 99.9% of
bacteria, including e-coli, salmonella,
H1N1 and MRSA.
No tools or experience are required to install
the door hand covers, which can be fitted in
under a minute.
Cherwell Laboratories Optimistic About Viable
Air Monitors
The company, together with Development Bank of
Wales, took a minority holding in Pinpoint
Scientific last September.
“Pinpoint is developing an exciting range of
viable air monitors for the pharmaceutical
industry. The ImpactAir range of air monitors
will directly address the need for continuous
monitoring within Grade A environments as per
the revised Annex 1,” said Managing Director
Andy Whittard.
For the Cherwell boss, growing demands around
environmental monitoring within pharma will
continue in 2020. “We see the ImpactAir product,
alongside our range of prepared media, as being
a key offering.
Redditch Medical has New Hand Hygiene Product
Redditch Medical has launched its hand hygiene
range in a time of greatest need. As the
Coronavirus pandemic continues to effect daily
life with an imminent release from "lock down"
expected and an expected increase in PPE and
Infection Prevention required Reddtich Medical
is prepared to support.
With two formulations in the range a hand rub (InSpec
HR) and a hand gel (InSpec HG). The hand rub is
manufactured according to the World Health
Organization formula and is available in a
convenient personal spray bottle. The Hand Gel
is specially formulated to provide fully
viricidal efficacy and excellent skin
compatibility with provitamin B5, aloe vera and
glycerol designed to protect the skin during
use.
With the development of this new range Redditch
Medical has added further investment into the
InSpec brand with new premises and filling
lines.
Sweden With No Lockdown Has Had Too Many Deaths
Sweden's controversial decision not to impose a
strict lockdown in response to the Covid-19
pandemic led to too many deaths, the man behind
the policy, Anders Tegnell, has acknowledged.
Sweden has seen a far higher mortality rate than
its nearest neighbors and its nationals are
being barred from crossing their borders.
Dr Tegnell told Swedish radio more should have
been done early on.
"There is quite obviously a potential for
improvement in what we have done."
Sweden has counted 4,542 deaths and 40,803
infections in a population of 10 million, while
Denmark, Norway and Finland have imposed
lockdowns and seen far lower rates.
Denmark has seen 580 deaths; Norway has had 237
deaths and Finland 321. Sweden reported a
further 74 deaths on Wednesday.
230,000 Healthcare Workers have Contracted COVID
More than 230,000 health workers around the
world have contracted the novel coronavirus
since the start of the global pandemic, while
over 600 nurses have died from it, according to
a new analysis by the International Council of
Nurses.
The figures show that an average of 7% of all
COVID-19 cases worldwide are among health care
workers.
The International Council of Nurses, which
represents more than 130 national nursing
associations with 20 million members worldwide,
said the analysis is based on data from its
associations, official figures and media reports
from a limited number of countries," since
"there is no systemic and standardized record"
of the global number of nurses and health care
workers who have tested positive for COVID-19 or
succumbed to the disease.
Fibre Extrusion Technology Says There May Be
Better Answers Than Polypropylene for Meltblowns
Fibre Extrusion Technology (FET), a UK-based
specialist in process solutions and equipment
for the manmade yarns and fiber extrusion
industry, has received unprecedented inquiries
about its nonwoven meltblowing systems since the
onset of the coronavirus crisis.
“We’re currently running trials, preparing
samples and defining specifications for
companies in Germany and Italy, as well as the
UK, and we could already have sold the lab line
we have here many times over,” said Managing
Director Richard Slack. “It’s primarily designed
for R&D and pilot scale applications, but trials
have proven it to be suitable for the low volume
production of critical meltblown face mask
materials. Some of the customers to whom we’ve
supplied similar lines have already pivoted
their production to this, which has generated
further interest.
“We feel, however, that we are ideally placed to
offer services to nonwoven companies who may be
exploring alternatives to polypropylene in
meltblown, due to our experience in working with
such a wide range of fiber types.”
FET’s meltblown system was originally developed
for companies looking to process high melt
viscosity medical grade resorbable polymers such
as polyglycolic acid (PGA), polylactic acid
(PLA) and polyhydroxl btyrate (PGH), mainly for
use in implantable products and other medical
devices.
The key applications for these fibers are in
hernia repair patches, staple reinforcement
buttresses, artificial skin, adhesion barriers
periodontal and ringival repair materials and
those for tendon and ligament repair.
“Our meltblowing system provides medical
companies and others dealing in such fibers with
a simpler processing route than other techniques
such as needle punching and a wide range of
structural and mechanical properties is
obtainable from batch production,” Slack said.
“There are also numerous options for
post-processing of the webs, by calendaring,
point bonding or lamination.”
Performance polymers such as TPU polyurethanes
and TPE thermoplastic elastomers are also
processed by a number of leading sportswear
companies on FET meltblown systems, while
engineering polymers such as ABS and PEEK, as
well as polycarbonate and halogenated polymers,
are other possible raw materials.
It is in the area of sustainable resins,
however, that FET believes much more can be
achieved.
Meltblown polypropylene nonwovens are the
critical component of the face masks needed for
Covid-19 frontline workers and their scarcity on
the open market has in part been the reason for
the reported shortages around the world.
An estimated 40 million face masks and other
disposable nonwoven-based PPE items are
currently estimated to be being consumed each
day, amounting to a daily 15,000-ton mountain of
waste — much of which must be incinerated.
“We’ve done a lot of work with sustainable
polyamides and polyesters, as well as with PHAs
and a range of of PLAs,” Slack says. “In the
longer term, there has to be a more sustainable
option than polypropylene in these products and
the opportunity to explore potential
alternatives — drawing on the know-how from the
extensive body of tests and trials we’ve carried
out in the past, as well as the machines run
commercially by our customers — is something I
believe makes us pretty unique in the services
we can offer nonwovens manufacturers.
Conventional meltblown and spunbonded systems
are usually designed for high capacity systems
and are not suitable for product development, he
adds.
“They consume high quantities of materials and
as a consequence are not suitable for
development work with high value materials or
for niche applications. They also rely on
specially formulated low viscosity polymers
which is a further limitation which does not
apply to us.
In processing finer filaments, FET has achieved
structures with average mean filament diameters
of 1.68 microns and 58% of between 0.5 to 1.5
microns, in web thicknesses of 37 microns with
bulk density of 98 mg/ml and porosity of around
92%.
FET’s system is designed for the processing of
pure polymer with no need for processing aids or
additives.
“A wide range of structural and mechanical
properties are obtainable, with numerous options
for post-processing of the web, such as by
calendaring, point bonding or lamination,”
Richard Slack concludes. “More effective and
sustainable PPE solutions could well be achieved
through further product development.”
NXTNano has Nanofiber Media Available for Face
Masks
A recent independent test by a mask supplier
showed high efficiency for media made by NXT
Nano. The company currently has some capacity
available for manufacturing N95 rated face mask
material. However they released this statement
“Please understand this situation is fluid, and
that as COVID-19 continues we expect this
capacity to fill”.
Materials are nanofiber coated PET in ranges
from 29 to 70 GSM depending on the needs of
individual manufacturing lines.
“As we continue to see very strong demand in nanofiber air filtration medias as well as our apparel, medical, and microfiltration business segments, we felt it was critical to keep capacity ahead of demand so our customers can continue to count on the fast order turns they have become accustomed to,” says director of sales Andrew McDowell.
