
Coronavirus Technology Solutions
May 29, 2020
San Francisco Now Requires Masks in Any Public
Place
Lydall has Multiple COVID Initiatives Including
Long Term Meltblown Contract with 3M
Meltblown Price Still High
Europe will Increase Meltblown Production from 17 tons per day in March to 51 tons per day in December
Sciessent Antimicrobial Used in Hanesbrands Masks
Teho Filter Using Ahlstrom Media for N88 Masks in Finland
Automation is a Partial Solution to COVID in
Meat Processing Plants
Some Move Toward
Mechanization in the U.S Meat Industry
Twenty-five High Speed Surgical Mask Lines
Ordered in Italy
AAF Advises Consideration of Membrane ULPA
Rather Than Microfiberglass
Jacob Holm Partnering with Under Armour
Battelle H2O2 System is Now Thought to be Good
for Only Three Mask Uses
___________________________________________________________________________
San Francisco Now Requires Masks in Any Public
Place
The City of San Francisco is now requiring that individuals wear masks whenever they leave their homes and interact with people outside their households. The city previously only required masks in mostly indoor situations, but it now mandates that individuals wear masks when exercising less than 30 feet from others or when passing people on the sidewalk.
During a Thursday press conference, Mayor London Breed stated that businesses have the right to turn away any individuals who are not wearing masks but asked that individuals not confront non-mask-wearers.
Lydall has Multiple COVID Initiatives Including
Long Term Meltblown Contract with 3M
Lydall actions relative to COVID were presented
along with 1st quarter results
earlier this month. “When it first became
apparent that COVID-19 would have a significant
impact on the global economy, we acted rapidly
and decisively to safeguard the health and
safety of our global workforce and the
sustainability of our business,” Sara A.
Greenstein, President and Chief Executive
Officer, said. “We immediately responded to the
large unmet need and global shortage of supplies
for front line and first responder personnel and
re-prioritized our manufacturing capabilities in
North America and Europe to produce filtration
products used in N95 respirators, surgical and
medical masks, and medical wipes, pads and
gowns. In response to our automotive customers
ceasing operations in the U.S. and Europe late
in the quarter, we quickly ramped down
production at our Thermal Acoustical Solutions
facilities in these geographies.”
Ms. Greenstein continued "Lydall's mission is to
create a cleaner, quieter, and safer world as we
have been doing for the past 150 years. COVID-19
highlights the enduring role the Company has
played in delivering life-saving capabilities in
specialty filtration. As experts in filtration,
we have been in regular contact with the highest
levels of the U.S. government, and in contact
with leaders in Canada, Europe, and the United
Kingdom to provide solutions and expertise to
help in the fight against COVID-19. "In response
to the global shortage of personal protective
equipment (or PPE), we have re-deployed people
and assets, and have significantly increased
production of filtration materials.
“In May,
we secured a major long-term agreement with
Honeywell to supply meltblown filtration media
for their N95 mask production facilities. Our
proven technical and production capabilities
were key factors in our selection. As a result,
we have already committed additional capital to
acquire a new meltblown production line to
satisfy this and related demand. We also
recently developed a new application for
nonwoven materials used in medical gowns and
secured an order from the New York Department of
Health for this product. "April volumes for
Performance Materials' Filtration subsegment
increased 20% compared to the prior year,
reflective of the demand for PPE. April volumes
in the TAS business were down almost 90%.
Our China sites were back in operation in the
first quarter of 2020, while our European
automotive sites have started to slowly ramp up
to support customer requirements. In North
America, Ford, GM and FCA have announced plans
to resume production on May 18. We are ready to
support this re-start and have completed a
reduction in force program in TAS to provide a
leaner fixed cost structure as demand comes
back.
"In summary, despite substantial headwinds in
the quarter, we reacted quickly to this crisis,
delivered sequential margin expansion and strong
cash flow, and have enhanced our liquidity.
Lydall's long history and product application
expertise as a trusted supplier of specialty
filtration solutions will be a cornerstone of
our long-term strategic vision, with the current
crisis accelerating our focus on our filtration
and engineered materials businesses."
Meltblown Price Still High
One Hong Kong trader was recently offering to pay €100 per kilogram – ten times the pre-crisis price, Pierre Wiertz, head of EDANA, the European Nonwovens and Disposables Association, told the press.
Sanne van der Lugt, a China researcher at the
Dutch Clingendael Institute, added that the
price of meltblown in China now stands at
Rmb400,000 ($56,500) a ton – twenty times its
pre-crisis price. China is making two million
N95 standard protective masks a day and could be
making a lot more, but for its shortage of
meltblown material.
Europe will Increase
Meltblown Production from 17 tons per day in
March to 51 tons per day in December
Europe will increase meltblown production by 34
tons per day in the next 7 months. Compare this
to Sinopec who built 10 lines for 18 tpd
production in just 4 months. This would give
Europe
capacity to produce 50 million surgical
masks or 15-20 million N95 masks per day. There
are 741 million people in Europe.
If 500 million people need a new mask
every five days, then Europe needs to produce
100 million masks per day. Assume that high
efficiency masks will be needed. In this case
Europe will only be able to supply 20% of its
needs by year end.
Innovatec, a family-owned firm based in
Troisdorf, Germany, is the largest producer of
meltblown fabrics in Europe, estimated to have
more than 50% of the continent’s capacity.
’I’d never have thought meltblown could become such a prized commodity,” Christian Klöber, Innovatec’s owner, told the FT. “The prices some Asian buyers are offering us are just eye-watering.”
The company is well-placed to meet the rising
domestic demand for materials, having ordered a
new meltblown production line last year and
after the coronavirus crisis broke, investing in
two more. This will enable it to cover 85% of
German demand and enough for four billion face
masks per year.
While Germany has not made its own masks in the past, relying on imports from China and elsewhere, the German government has now put the entire industry out to tender, guaranteeing prices until the end of 2021.
Around 50 German companies have secured a place
on the scheme to produce ten million specialized
N95 masks and 40 million operating room standard
masks a week from August.
Also based in Troisdorf, is Reifenhäuser Reicofil, the leader in meltblown production technology. Around 75% of all hygiene and medical nonwoven fabrics worldwide are estimated to be made on Reicofil lines.
“When you start thinking how many production lines will be needed to meet demand, your head starts spinning,” said managing director Bernd Kunze. “We have been inundated with orders from Europe, Asia and the US and have dramatically increased our delivery frequency in response. Before, it would take us at least eight to nine months to supply a production line, now we’re doing it in three-and-a-half to six months.”
Reicofil will benefit from a new subsidy regime being put together in Berlin, under which the government will cover 30% of the cost of a meltblown production line, as long as the manufacturer pledges to sell exclusively into the German and European market by the end of 2023.
EDANA estimates that Europe is set to triple its
output of meltblown between March and the end of
the year, from 500 tons a month to 1,500 tons.
Sciessent
Antimicrobial Used in Hanesbrands Masks
Sciessent has partnered with both healthcare and
non-healthcare manufacturers to develop and gain
regulatory clearance for masks containing Agion
Antimicrobial for use in healthcare settings.
Sciessent has helped two suppliers obtain
approval for
the anti-microbial masks—one with a
traditional medical device manufacturer
following the severe acute respiratory syndrome
(SARS) outbreak in the early 2000s, and another
this year with a manufacturer from outside of
the medtech industry—comparing and contrasting
the two from an FDA regulatory process
perspective.
Following the 2003 SARS outbreak, medical device
manufacturer Nexera made the decision to develop
a N95 respirator mask constructed from Foss
Performance Materials’ Agion Antimicrobial
treated polyester fiber. As a medtech company,
Nexera had experience with FDA review for its
devices so it understood what the process
entailed. The company looked to Sciessent as its
supply partner to help with regulatory
considerations related to the antimicrobial.
Because Nexera had developed the mask in
response to SARS, as a product that could help
in future respiratory virus outbreaks, the
Nexera and Sciessent teams had ample time for
pre-planning prior to FDA submission. This
included the opportunity to develop data to
support the mask’s antimicrobial claims. Working
together, the teams successfully obtained FDA
clearance for Nexera’s SpectraShield 9500 N95
respirator mask, and later leveraged additional
data to secure an updated 510(k) with cleared
claims to inactivate viruses by 99.99 percent in
five minutes.
Sciesent also worked with Hanesbrands. While the
FDA is leveraging its Emergency Use
Authorization (EUA) to accelerate the timeframe
for clearance of products to address the
COVID-19 crisis, and this is certainly a benefit
to companies producing PPE, Hanesbrands still
had to meet the agency’s requirements. Sciessent
served as a collaborative partner in these
efforts with medical device expertise, a wealth
of data, and extensive experience in navigating
the FDA’s regulatory review pathway.
Unlike the premeditated work with Nexera to
develop a mask following the SARS outbreak
nearly two decades ago,
work with Hanesbrands required that the
team leverage existing knowledge and experience
to quickly fill-in various information gaps.
Instead of developing a product from the ground
up, Hanesbrands was repurposing its knitted
cotton fabrics treated with Agion Antimicrobial
to develop its masks.
Sciessent supported Hanesbrands in their
interactions with FDA. Given the urgent need to
address the PPE shortage, it appeared
communication was far more open and frequent
with the agency under the EUA directive. The
correspondence and exchange of information
between Hanesbrands and FDA moved quickly. In
this circumstance, the FDA did not clear
specific efficacy claims; in fact, the agency
said no efficacy claims could be made.
Having worked closely with the FDA with Nexera
for its N95 mask and with other medical device
manufacturers for their products, Sciesent
was able to pivot quickly and apply its
understanding of the regulatory process to the
Hanesbrands situation. It was also able to
leverage the data on safety and efficacy,
including viruses from the Nexera mask’s second
510(k) clearance, to help fast track the
Hanesbrands mask clearance. As a result, FDA
cleared Hanesbrands’ mask in a matter of days,
enabling the company to quickly transition its
operations to relieving the PPE shortage.
Teho Filter Using
Ahlstrom Media for N88 Masks in Finland
Ahlstrom-Munksjö is supplying facemask material from its plant in Tampere to Teho Filter for the assembly of masks. The masks will be available in May-June in the stores of Finland based retailer S Group. The filtration efficiency of the face mask material of 88% has been verified by VTT Technical Research Centre of Finland. It is substantially higher than the roughly 20-40% efficiency of masks made from cloth; the company claims. The filtration efficiency of a mechanical filter media remains intact over time compared to electrostatically charged materials the efficiency of which may decrease in humid conditions, Ahlstrom-Munksjö adds.
“We work with determination in order to enhance
the supply of face masks for consumers in
Finland. At the same time, we are further
supporting the reopening in Finland once the
corona pandemic restrictions loosen,” states
Otto Kivi, Sales Manager at Ahlstrom-Munksjö’s
Filtration business.
The company announced in April that it would start the production of facemask materials in Tampere, where its plant is capable of producing material for more than 10 million masks in the short-term and has the ability to increase capacity to about 30 million masks per month. Earlier in May, the company announced a similar co-operation on facemask supply with other Finnish players.
” We are also very pleased about the continued product development in Tampere: our production now meets the filtration efficiency requirements of surgical face mask materials. Currently, we are working together with our customers to develop a product that meets the European Standard EN 14683 for surgical masks. The availability of various protective gear is vitally important,” Kivi continues.
Ahlstrom-Munksjö has long-term and in-depth
knowledge in the production of protective
materials for the healthcare industry. Due to
the coronavirus pandemic, the company has
increased its offering in protective fabrics and
expanded the production of face mask materials
globally to support the healthcare sector.
Automation is a Partial Solution to COVID in
Meat Processing Plants
Inside Europe’s largest pig slaughterhouse, the
only visible sign that there’s a global pandemic
going on is in the break room, where every other
chair has been spirited away to leave
conspicuous gaps between any would-be
socializers. Otherwise, it’s business as usual.
That’s because, at this meat plant, robots do
most of the work.
The operations were described in a recent
Wired article. Automated partitions nudge a
few pigs at a time, out of the pens and into a
gas chamber where a blast of CO2 knocks
them out. Moments later, they spill onto a
conveyor belt where a worker wearing a
waterproof apron and elbow-length gloves cuffs
one of each pig’s rear feet to a moving
production line, which hoists the animal
overhead. Another worker inserts a knife into
the pig’s carotid artery, and an attached vacuum
hose siphons out the blood. That’s when the
robots really take over.
An infrared laser-emitting robot first measures
each pig carcass. Next up, the so-called rectum
loosener robot uses computer vision to identify
the pig’s tail, cuts a 4-inch hole around it,
and extracts whatever poop is inside. Then the
feces-free carcass moves into a cabinet-like
robot, where a large, circular blade splits the
pig from sternum to ham. Next, each one moves
onto a mechanized, autonomous organ remover,
tendon slasher, and finally, the spine splitter.
Ten minutes. Six robots. Minimal human
supervision. By midnight, when the second
(human) shift calls it quits, 18,000 pigs will
have passed through this gauntlet of actuated
steel and knives.
Danish Crown’s Horsens facility isn’t just one
of the largest pig slaughterhouses in the world,
it’s also, by most accounts, the most modern.
(And the most transparent—in pre-pandemic times,
it hosted hundreds of visitors a week. Today you
can still take a
virtual tour.)
But heavy automation is a feature of all 18 of
the company’s in-country meat processing
facilities. And it’s one reason that might
explain how Denmark’s slaughterhouses have so
far escaped becoming Covid-19 hot spots.
According to a Danish Crown spokesperson, among
the company’s 8,000 employees in Denmark, fewer
than 10 workers have tested positive for the
novel coronavirus. None of its slaughterhouses
there have had to close or slow down production.
There are likely other explanations and
contributing factors, too—like Denmark’s early
adoption of lockdown measures and its robust
nationalized health care system. But scientists
who study the meat industry say the rest of the
world should take note. The new realities of
social distancing mean rethinking
the layout of
all kinds of workplaces, including
slaughterhouses. In the US, these facilities are
characterized by cramped, loud, icy conditions that
make it easier for
the coronavirus to stay alive and jump from
person to person. Robots could
help keep workers safe and meat plants running.
Some Move Toward Mechanization in the U.S Meat
Industry
In the U.S., poultry production has been getting
steadily more mechanized for decades—going from
3,000 chickens processed per hour in 1970 to
8,000 in 1980 and 15,000 today. The birds’
smaller bodies mean companies need less capital
investment to automate their production lines.
But it’s only in the past 10 years or so, says
Shai Barbut, a professor of meat science at the
University of Guelph in Ontario, that pork and
beef processors have started to catch up. In
2018, a pork plant opened in Coldwater,
Michigan, with automated cutting and packaging
robots that enabled the company that operates
it, Clemens Food Group, to produce the same
volume of pork with 300 fewer workers. Tyson
Foods also began
investing in
robots for its pork plants, primarily to combat
labor shortages.
Getting robots to do the job of human butchers
isn’t trivial. Like lettuce and apples,
animals come in all shapes and sizes. And though
farmers can try to make them as genetically
similar as possible and feed them the same
amount of food, two pigs will never be identical
in the way two smartphone
batteries are.
“https://www.wired.com/story/covid-19-makes-the-case-for-more-meatpacking-robots/
Twenty-five High Speed Surgical Mask Lines
Ordered in Italy
Fameccanica.Data S.p.A,
a joint venture between Angelini Holding
and
Procter & Gamble,
recently launched the Fameccanica Protective
Mask machine (FPM).
The machine was at the center of an agreement
between Fameccanica and the Italian Government
for the fight against the propagation of
Coronavirus (COVID-19), signed on May 2. The
agreement provides for the supply of 25
high-speed production lines for surgical masks.
"This project is based on an extraordinary
industrial, technological and human commitment
and was born in a logic of service to the
country in a moment of extreme difficulty. We
were quick to design an innovative technology
which combines speed standards with the
product's ability to overcome the limits of
finding raw materials," comments Alessandro
Bulfon, general manager of Fameccanica. "This
puts us in a position today to face the
country's reopening phase and tomorrow to deal
any other emergency health situation."
The FPM machine places Italian technology and
initiative at the service of the emergency both
for Italy and for other countries in the world.
The high speed and ability to process a wide
range of materials, as a matter of fact, can
respond widely to the internal demand for
surgical protective devices, making the national
production chain independent.
Technology:
• Fastest machine in the world with over 45,000
mask/hour production speed (800 pieces/min)
• Fast and easy product size change
• Short-time installation and start-up
• One-operator process
• Patented product, process and technology
• Energy consumption optimization
AAF Advises Consideration of Membrane ULPA
Rather Than Microfiberglass
Particles that are 0.5μm in size or smaller tend
to follow increasingly erratic paths as particle
size decreases, a phenomenon known as the
diffusion effect. As such, HEPA and ULPA filters
are often rated according to their
most penetrating particle size, or the size of
particles that most readily pass through
them. As depicted in this line graph, filters
that achieve the same efficiency rating, in this
case ULPA filters rated at 99.999%, are not
necessarily equal in their MPPS performance.
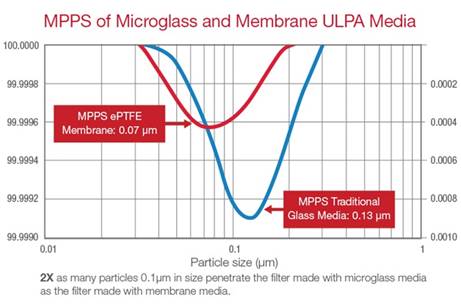
Microglass media filters still have their place,
such as high-temperature applications. However,
membrane media offers compelling reasons to make
a switch in HEPA and ULPA filter media.
-
When purchasing HEPA and ULPA
filters from AAF Flanders, media
production, testing, and
packaging are all performed in
an ultra-modern ISO-Certified
controlled environment,
eliminating the potential for
contamination during the
manufacturing process.
-
Microglass media frequently
suffers damage during shipping,
handling, installation, and
testing, leaving
cleanroom operators exposed to
contamination risks from leaks
that may escape the attention of
the naked eye. Membrane media
clearly outperforms microglass
media in terms of tensile
strength, abrasion resistance,
and burst pressure.
-
Membrane media offers a lower
differential pressure than
microglass media. Not only does
this trait improve the energy
efficiency of HVAC equipment,
but it also reduces the wear and
tear on this equipment.
-
Jacob Holm Partnering with Under Armour
Jacob Holm, the manufacturer of Sontara
nonwoven fabrics in Nashville, TN, partnered
with global sports performance brand Under
Armour headquartered in Baltimore, MD, to
produce much needed personal protective
equipment including face masks and isolation
gowns. The partnership has involved more than 50
Under Armour teammates who created a new mask
design and contributed to a 65% increase in
Sontara production since March.
Sontara has been creating medical-grade fabrics
for more than 45 years. In the last month, they
have seen a multifold increase in demand for
healthcare fabrics, requiring a 65% increase in
production in March over their 2019 projections
and leading to the hiring of 67 new production
employees.
“This situation
is unprecedented," says CEO Martin Mikkelsen.
"However, for Jacob Holm as a company, the only
way forward is to lean in to what we know and
use the full force of our experience to help
contain the spread of COVID-19 and make a
positive impact on the well-being of our
communities.”
As proof of this
effort, two of Jacob Holm’s five manufacturing
sites reported record production volumes last
month. To support this effort, Jacob Holm hired
relatives and friends of current employees who
were furloughed or laid off.
Under Armour is now converting Sontara material
into its one piece, no sew mask design. The mask
acts as a first level of defense, reducing virus
laden moisture and droplet spread from the
wearer, and preventing face touching by the
wearer.
Sontara has increased mask and gown production
partnerships across the U.S. and Europe and has
donated the equivalent material of well over a
million masks through Spain and France.
Battelle H2O2 System is Now Thought to be Good
for Only Three Mask Uses
It sounded like a great deal: The White House coronavirus task
force would buy a defense company’s new cleaning
machines to allow critical protective masks to
be reused up to 20 times. And at $60 million for
60 machines on April 3, the price was right.
But over just a few days, the potential cost to
taxpayers exploded to $413 million, according to
notes of a coronavirus task
force meeting
obtained by NBC News. By May 1, the Pentagon
pegged the ceiling at $600 million in a
justification for
awarding the deal without an open bidding
process or an actual contract. Even worse,
scientists and nurses say the recycled masks
treated by these machines begin to degrade after
two or three treatments, not 20, and the company
says its own recent field testing has only
confirmed the integrity of the masks for four
cycles of use and decontamination.
Nurses in several places across the country now
say they are afraid of being at greater risk of
acquiring COVID-19 while using N95 masks, which
they say often don’t fit correctly after just a
few spins through a cleaning system that uses
vapor phase hydrogen peroxide to disinfect them.
NBC has gathered information on problems with
the task force’s methods. Working without
external oversight, it has pumped billions of
dollars into hard-to-trace contracts for
COVID-19 supplies that often don’t pan out as
advertised.
Battelle stands by its 2016 study of its
technology, which used manikins rather than
human subjects to determine whether masks lost
their fit or were permeated by particles after
20 uses, according to company officials who
responded to NBC News’ inquiries in an email.
But the company also said it has only verified
the purity of masks for four uses in field
testing at Massachusetts General Hospital since
the machines were built to respond to a
pandemic. That puts health care workers in the
position of being the first living experimental
test subjects.
“To date, Battelle has received and tested
samples representative of four actual use cycles
from MassGen,” Will Richter, Battelle’s
principal research scientist, said. “The goal of
this assessment is to determine the impact of
actual wear.”
Battelle’s sanitizers were mobilized by a task
force designed to execute on Trump’s demands,
despite reservations about safety and cost.
Technically, the Defense Logistics Agency, an
arm of the Pentagon working with the task force,
gave Battelle a “contract letter,” which allows
for details of a deal to be finalized after the
work starts. When DLA officials submitted a
legally required justification explaining the
parameters of the deal this month, they wrote
that the "maximum dollar value" is now $600
million.
The company says it might not hit the cap.
“As demand ebbs and flows at various sites
across the country, Battelle will adjust its
staffing accordingly and will bill the
government only the actual costs incurred,”
company spokesperson Katy Delaney said. “If the
contract costs are less than the ceiling cost,
then the government will not spend up to the
ceiling.”
DLA spokesman Patrick Mackin said the $187
million of extra room is there for flexibility."
To date, the value of the contract remains at
$413M," he said in an email. "The maximum value
of the contract is $600M in the event we need to
make any adjustments in the support provided by
Battelle during the period of performance."
The task force’s deployment of mask sanitizers,
several other versions of which have been given
an emergency greenlight since Battelle’s went
into service, are now part of a transition to a
focus on boosting the economy, because the
administration insists, they reduce the need to
supply fresh masks to health care workers. The
president himself has said workers have all the
equipment they need.
When task force leaders convened at FEMA
headquarters on April 8, they faced a conflict
over whether to proceed with Battelle’s contract
despite the sharp price spike.
Trump clearly wanted the mask sanitizers to be
deployed rapidly. It had only been 10 days since
he tweeted his support for the
FDA waiver, which
allowed masks cleaned by the machines to be used
in health care facilities and freed the company
from existing federal quality-assurance
regulations.
But from April 3 to April 8, the price had
skyrocketed from $60 million to $413 million. An
Ohio-based nonprofit corporation that pays top
executives more than $1 million a year and spent
$350,000 lobbying Congress and federal agencies
from Jan. 1 to March 30, Battelle raised the
price for each machine from $1 million to $6.8
million “due to the inclusion of operating costs
for six months, shipping, and logistics tails to
be covered up front,” according to a summary of
the decision-making meeting that was circulated
to task force members and obtained by NBC News.
The “logistics tail,” a term the military uses
to describe the chain of goods and people
supporting combat troops in war, broadly refers
to the costs of providing supplies and
administrative support for a project. The
additional $353 million over six months for the
logistics tail, which includes the price of employing
and training technicians,
is equivalent to
the retail value of 278 million new N95 masks.
In addition to operating the machines,
maintaining them and shipping masks back and
forth to health care systems, Delaney said “each
site requires things like portable restrooms,
showers, protective equipment and in some cases
very large tents to house the operations.”
Five days after the deal became public, an NIH-led
study concluded
that the hydrogen peroxide vapor method of
decontamination is only safe for three cycles.
The study, conducted out by the National
Institute of Allergy and Infectious Diseases,
which is run by Dr. Anthony Fauci, used
different methods than Battelle’s, according to
Dr. Seth Judson, a University of Washington
internal medicine resident who worked on the
evaluation. The NIH version employed special
technology to measure exposure of the virus
inside masks and tried to replicate how they
would maintain their fit on real people, as
opposed to the manikins used in Battelle’s
study.
Battelle’s system is already in use by over 400
hospitals across California alone, according
to state records,
and several other companies have won FDA waivers
to deploy mask-sanitizing machines since
Battelle was granted its exemption.
