
Coronavirus Technology Solutions
May 15, 2020
Cleamix Oy Supplies H2O2
Decontamination for Korea Center
for Disease Control
Vaisala Analyzer for H2O2,
Temperature and Humidity
Ontex Constructing
80 Million Mask per Year
Plant in Belgium
Ultra-Pure PM 0.1 Mask Filter
from Asiatic Fiber Corporation
Orum International has New and
Improved Microbiological Sampler
New Isolation Modules for
Healthcare Facilities
Perkins and Will Helps in
Analyzing Facility Capabilities
M+A Architects Helps Hospitals
Install Additional Filtration
HGA Designing Prefabricated
Modular Patient Rooms for Four
Facilities
American Institute of Architects
Launches Pandemic Task Force
HDM Global has Anti-Microbial
Coatings for Floors, Furniture
and Textiles
___________________________________________________________________________
Cleamix Oy Supplies H2O2
Decontamination for Korea Center
for Disease Control
Recent bio-decontamination work
performed by
Cleamix Oy
at Korea’s Centers for Disease
Control has validated hydrogen
peroxide vapor
bio-decontaminations in early
2020 during the coronavirus
outbreak. The Cleamix
bio-decontamination units are
portable, highly efficient
hydrogen peroxide vapor
generators. The generators use
Vaisala’s HPP270 series probes
to automatically control vapor
output during
bio-decontamination. The probes
also provide stable, accurate
monitoring data that allows
operators to observe process
conditions in real-time.
Cleamix CEO Panu Wilska
described the company’s work
with Korea’s Centers for Disease
Control (KCDC) in the wake of
the coronavirus outbreak.
“The
KCDC has Biosafety level 2 and 3
laboratories with a total volume
2500 m3,” says Wilska. “Our
bio-decontamination contract
with our local partner BioAll
included both laboratories. The
labs were about equal in size,
with multiple separate rooms,
airlocks and corridors. To
decontaminate the spaces we used
four portable networked Cleamix
vapor generators.”
Biosafety laboratories are used
to study contagious materials
safely; with protective measures
for personnel and to prevent
contamination. Biosafety labs
are designed and operated in
compliance with laws, policies,
regulations, and guidelines for
research into infectious agents.
This research is needed to
understand pathogens in order to
produce vaccines and other
treatments.
There are four levels of
biosafety that define the type
of research that can be
performed and the safety
measures that must be employed.
These levels are based on the
practices, processes and systems
that provide protection from the
pathogens being researched. From
BSL-1 to BSL- 4, the protective
barriers and processes increase.
Biosafety level 1 covers work
with microorganisms that present
a minimal threat. Biosafety
level 2 laboratories research
agents with a moderate risk.
Extra cautions and protections
are used, with added constraints
on access and processes. BSL-2
labs use biosafety cabinets and
other containment systems.
During the bio-decontamination
process, the HPP270 probes
showed that the H2O2
concentration was rising quite
rapidly,” says Wilska. “The
average treatment time per
segmented area was seventy-five
minutes, plus aeration time.
Aeration was very fast as we
could have the air conditioning
system turned on after each
treatment. The process was
validated by biological
indicators to have achieved a
6-log kill.
Vaisala Analyzer for H2O2,
Temperature and Humidity
A new innovation is
called PEROXCAP®, for measuring
vaporized hydrogen peroxide,
temperature, relative saturation
and relative humidity. The
PEROXCAP® sensor is
implemented in the HPP270 series
probes that are intended for
ecological hydrogen peroxide
bio-decontamination
applications.
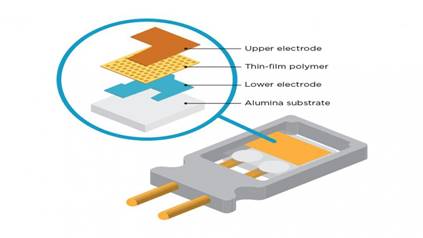
This unique technology enables
accurate and repeatable
measurement of the
bio-decontamination cycle with
one single probe.
|
Benefits |
|
Repeatable measurement |
|
Excellent long-term stability |
|
In addition to H2O2 ppm measurement, the sensor also measures humidity and temperature when combined with an additional temperature sensor |
|
Tolerates high humidity and measures accurately even in condensing conditions |
|
Long lifetime of the product |
|
Accurate measurement with a traceable H2O2 factory calibration |
|
Long annual calibration interval |
|
Easy on-site calibration |
Ontex Constructing
80 Million Mask per Year
Plant in Belgium
Ontex will
start the production of face
masks by September 2020, with a
capacity of around 80 million
IIR-type surgical face masks per
year, the Belgium-based hygiene
product manufacturer has
announced.
“We invest in
the production of surgical face
masks to support caregivers and
other essential workers,” said Annick
De Poorter, Ontex’s vice
president for R&D, Quality and
Sustainability. “We want to help
protect these people, including
Ontex employees, who keep
society going by providing
essential goods and services.”
The surgical face masks of the
IIR type, which are typically
used in hospitals, are planned
to be produced on a line at
Ontex’s factory in Eeklo,
Belgium in accordance with
applicable regulations. Ontex
has ordered specialized
machinery and is training staff
to get certified for production
and start producing face masks
in September or earlier. The new
production is Ontex’s own
initiative and receives no
government funding.
In order to offer face mask
sourcing options for caregivers
and other essential workers
before September, Ontex is
providing local authorities a
direct contact with a trusted
face mask supplier which it is
using as well. Ontex provides
this assistance to the extent
allowed, as some governments
have strict regulations whereby
only their competent
administrations are entitled to
coordinate the sourcing of
protective gear against
COVID-19.
Ultra-Pure PM 0.1 Mask Filter
from Asiatic Fiber Corporation
The mask
filter has three layers, and
each layer has its unique
purpose. The outer layer is
air-droplets blocker, that can
preliminarily filter the
majority of particles and
air-droplets. The second layer
is an AFC® filter pad, it
embraces air-in area and air-out
valve, to create an PM 2.5
filtration effect. The base
layer is anti-bacterial layer,
and it also brings anti-odor and
anti-static effect (to diminish
the adherence of particle). The
anti-bacterial is a long-lasting
and durable for several times of
laundry. This mask filter is
being tested that can
effectively block, filter and
form an excellent barrier of
air-droplets, micro-particle,
bacterial, dust, pollen and smog

Features:
·
Triple filtration, BFE &
PFE ≥ 99%, tested by Nelson labs
·
PM 2.5 level, effectively
filtering micro-particle and
smog
·
Centralized air exchange area,
to enhance filtering effect
·
Exceptional air valve design,
brings comfort without
sweltering
·
Long-lasting anti-bacterial,
remains 99% even after 50 times
laundry
·
Multi-functional fiber, brings
anti-bacterial, anti-odor and
anti-static (less particle
adherence)*BFE = Bacterial
Filtration Efficiency; PFE =
Particle Filtration Efficiency
*PM = Particulate Matter
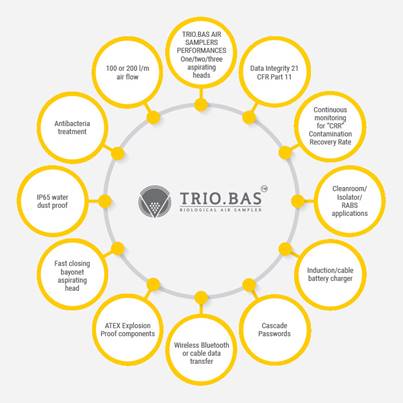
Orum International has New and
Improved Microbiological Sampler
In the 80’s the company
designed, created and patented
the first portable
microbiological air sampler
which became the reference tool
for monitoring the
microbiological contamination of
the air.
After creating the first
portable sampler, the Company
today introduces a new model the
TRIO.BAS (patented), taking
advantage of the gained
experience, together with the
new technologies and application
needs.
TRIO.BAS is a new portable
instrument, unique in the
market.

Features of Trio. Bass are
displayed below.
New Isolation Modules for
Healthcare Facilities
Rather than converting existing
buildings into patient care
facilities, some designers have
decided to create new systems
for emergency intensive care. CURA pods
are one such solution. CURA
(Connected Units for Respiratory
Ailments) is an open-source
design for emergency COVID-19
hospitals initiated by a team of
architects, engineers, doctors,
and military experts and
currently being built in Milan.
The pods use repurposed 20-foot
shipping containers turned into
biocontainment pods and are
easily deployable in cities
around the world. While each
unit functions autonomously,
individual pods can be connected
by an inflatable structure to
create modular configurations
and would contain all the
equipment needed for two
patients including ventilators.
The best part—they can be
deployed in just a few hours in
parking lots or as self-standing
field hospitals.
Jupe, Inc., a
flat-packed housing startup
built for crisis and disaster
response, has also developed a
mobile recovery unit in response
to hospital overcrowding. JUPE
Health are a series of three
recovery spaces designed for
both healthcare workers and
quarantined patients including
JUPE Rest (sleeping unit), JUPE
Care (recovery unit for
non-critical patients), and JUPE
Plus (a stand-alone intensive
care unit). JUPE Health can
deploy up to 24 units with a
40-foot flatbed and pickup truck
and up to 500,000 on a single
cargo ship. The efforts are led
by physician Dr. Esther Choo (of
#GetMePPE), designer Cameron
Sinclair, and modular housing
entrepreneur Jeff Wilson.
Perkins and Will Helps in
Analyzing Facility Capabilities
For Seattle-based Perkins and
Will principal Brad Hinthorne,
AIA, assisting his clients in
the Pacific Northwest—one of the
first regions in the U.S. to
experience a surge of COVID-19
patients—had little to do with
design plans or construction in
the early phases. Instead,
hospital systems needed help
organizing incoming data. “It
really started with helping them
get real-time data of how many
places they could accommodate
cases across their system,”
Hinthorne says. To address this
need, Perkins and Will created
dashboards with “graphics that
their C-suite could use in their
command center to help manage
the day-to-day work they were
doing,” he says.
Perkins and Will has also been
fielding calls from health care
clients “asking about how they
can rework their MEP systems to
make more patient rooms negative
pressure,” says Marvina
Williams, a registered nurse and
health care operations
specialist based in Perkins and
Will’s Atlanta office. “They're
looking closely at how they can
turn patient floors into
negative pressure floors for
really sick patients that have
COVID-19 symptoms.”
Some hospitals were designed
with the capacity to convert
emergency rooms and non-critical
care units to rapid-response and
intensive care units.
As an example, Williams points
to the Rush University Medical
Center in Chicago, which Perkins
and Will designed with
bioterrorism and surge
preparedness. At Rush, doors to
the ambulance bay can be closed
to create a COVID-19 triage area
to limit exposure to other
emergency room patients. In case
of a surge, the ER was designed
to be acuity adaptable with
structural columns fitted with
electrical outlets and to handle
medical gases. “The emergency
room is divided into three zones
and set up for cordoned-off
negative-pressure areas,”
Williams explains. “If 20 beds
are needed for negative
pressure, they can do that. If
they need 20 more, they can
continue on.”
However, the reality of
transforming or creating more
urgent-care facilities quickly
to address COVID-19 surges is
sobering. “Even if you wanted to
do it by hospital room, the
majority of the infrastructure
on a campus is not designed for
these types of loads,” Hinthorne
explains. Instead, cities and
hospital systems are setting out
to optimize existing
infrastructure and invest in
alternative spaces for less
critically ill patients.
“Creating critical care settings
outside of a hospital is
incredibly challenging,” Rogers
says. “We would get more value
from using non-hospital settings
for supporting lower acuity
care, for supporting
non-infectious care, for
supporting staff who need a
place to sleep.”
It is important to share the new
expertise with cities
experiencing later COVID-19
surges and for future design
projects. Perkins and Will’s
client Providence Health &
Services, which operates 50
hospitals in Alaska, Washington,
Oregon, California, Montana, New
Mexico, and Texas, is already
sharing relevant data and
lessons across their various
facilities.
M+A Architects Helps Hospitals
Install Additional Filtration
Elizabeth Koelker who works for
M+A Architects as its Director
of Healthcare and Higher
education studio has worked on
previous projects at Central
Ohio hospitals and ERs.
"We are dedicated to healthcare
architecture," said Koelker.
"They're really approaching the
preparation for the influx of
COVID-19 patients very
holistically and converting
existing medical, surgical care
rooms to ICU rooms is really
just one of the things they're
doing."
She said she's working with
Central Ohio Healthcare systems
to prepare for large waves of
patients. However, she would not
name which facilities she's
working with.
The work includes advising them
on restructuring ICUs and ERs,
using separate entrances and
elevators for COVID-19 patients,
creating separate wards and even
re-working HVAC systems.
"They are able to install
additional filtration to capture
any of the particles that may
get into the systems," said
Koelker.
She said it's a lot of work but
it's nothing our hospitals can't
handle. "They have plans that
they've put in place in the past
already to begin to understand
how to begin for surges like
this. Now it's a matter of
addressing it in a very short
period of time."
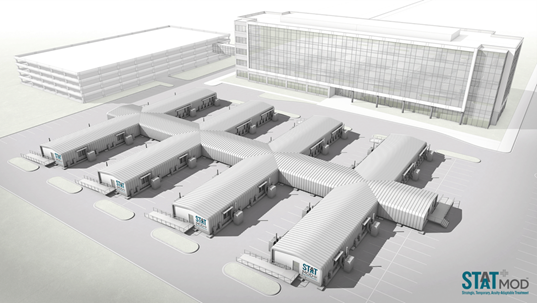
HGA Designing Prefabricated
Modular Patient Rooms for Four
Facilities
The Boldt Company, a contractor
in Appleton, Wisconsin, is
prefabricating modular
structures, designed by the firm
HGA, that meet the need for
speed without the sanitation and
ventilation problems of tents.
STAAT Mod, as the units are
called, can be delivered on a
standard truck trailer. They
contain two finished patient
rooms and a bathroom, along with
medical and HVAC infrastructure
that meet the negative-pressure
standards for AII rooms.
 .
.
Virtual reality simulations
allowed health-care
professionals and HGA process
engineers to weigh in on the
validity of the designs, even as
social-distancing orders
precluded in-person meetings and
the construction of physical
prototypes. Projects are
currently under way at four
sites, with the first 16-bed
configuration planned to be
operational the first week of
May.
American Institute of Architects
Launches Pandemic Task Force
The American Institute of
Architects has launched a task
force to consult on how to adapt
buildings into healthcare
facilities during the coronavirus
pandemic.
The initiative by American
Institute of Architects (AIA)
was created in response to
Covid-19 in the US, which
now leads the world in confirmed
cases.
The task force is intended to
provide information on how to
convert existing buildings into
temporary healthcare facilities
to treat those suffering from
the virus.
"On a daily basis, I am hearing
from our architects who feel a
deep sense of moral duty to
support our healthcare providers
on the frontlines of this
pandemic," said AIA president
Jane Frederick.
"As our communities assess
buildings to address growing
surge capacity, we hope this
task force will be a resource to
ensure buildings are
appropriately and safely adapted
for our doctors and nurses."
Kirsten Waltz, the director of
facilities, planning and design
at Massachusetts healthcare
nonprofit Baystate Health, is
working with the task force to
advise how to modify hospitals
and smaller facilities to meet
the surging demand of beds, as
well as design more medical
screening and triage areas.
"This is a race against time for
healthcare facilities to meet
bed surge capacity needs," Waltz
said. “This task force will help
inform best practices for
quickly assessing building
inventory and identifying
locations that are most
appropriate to be adapted for
this crisis."
Environmental health scientist
and architect Molly Scanlon, who
is chair of AIA's task force,
said that many more places are
needed to treat the number of
people that will be affected.
"During the Covid-19 pandemic
public health response there is
an unprecedented need for the
adaptive reuse of buildings to
serve a variety of functions,"
she said.
"Architects and our allied
design and construction
professionals are in a unique
position to leverage our
advanced problem-solving skills
to bring forth ideas for
community implementation."
The AIA is also developing a
Covid-19 report called the Rapid
Response Safety Space Assessment
for members to use to consider
the suitability of buildings and
spaces sites for relevant care.
HDM Global has Anti-Microbial
Coatings for Floors, Furniture
and Textiles
There are seven main areas
fielded by the Coatings
Corporation of HDM Global
Corporation. The coatings
provide sterilizing properties
against human pathogens. From
hygienic textile applications,
anti-viral epoxy flooring and
paints, to bedbug eradication,
fixed mold abatement, hands-on
training and more, HDM Coatings
Corp. is quickly surpassing its
mission to “Embrace Global
Purity.”
Main Areas of HDM Coatings are
-
Professional
corporate
training for
product
applications
-
Hygienic and
biocidal epoxy
flooring
additive
-
Textile coatings
including bedbug
and insect
eradication
-
Hygienic
transportation
industry
interiors with
anti-viral
properties
-
Safe and pure
offset printing
varnish inks and
prints
-
Fixed safe mold
and mildew
abatement
-
Pure, anti-viral
painting
applications:
Industrial,
Commercial and
Residential
Textile sprays are used to
protect interiors of
automobiles, trains, buses,
airplanes, and more. Our textile
spray will add a necessary,
hygienic, and anti-viral layer
of protection. HDM’s textile
sprays guard against bedbugs,
insects, and pathogens of many
types in any level of public or
private transportation.
