
Coronavirus Technology Solutions
May 4, 2020
Testing with Sartorius Filters in Singapore
Hospital Shows Virus Deposition in Vents
STARC Systems to Provide Critical Isolation
Rooms
Portafab Supplies Temporary Isolation Rooms
Healthmark Provides Tags, Labels, Trolleys for
Reprocessing N95 Masks but also Cooling
Accessories
Ortner Furnishes Fan Filter Units for Retail
Check Out
Ortner Furnishes Three Way Airlock H2O2
Decontamination System
Partitions Without Laminar Air Flow May Increase
Virus Transmission
Octanorm Supplies Partitions for a Large London
Emergency Hospital
Telstar’s Micro Biosafety Cabinets are Suitable
for the Manipulation of Samples with Biological
Risk Level 3
______________________________________________________________________________
Testing with Sartorius Filters in Singapore
Hospital Shows Virus Deposition in Vents
From January 24 to February 4, 2020, three
patients at the dedicated SARS-CoV-2 outbreak
center in Singapore in airborne infection
isolation rooms (12 air exchanges per hour) with
anterooms and bathrooms had surface
environmental samples taken at 26 sites.
Personal protective equipment (PPE) samples from
study physicians exiting the patient rooms also
were collected. Sterile premoistened swabs were
used.
Air sampling was done on two days using SKC
Universal pumps (with 37-mm filter cassettes and
0.3-μm polytetrafluoroethylene filters for 4
hours at 5 L/min) in the room and anteroom and a
Sartorius MD8 microbiological sampler (with
gelatin membrane filter for 15 minutes at 6 m3/h)
outside the room
Specific real-time reverse
transcriptase–polymerase chain reaction (RT-PCR)
targeting RNA-dependent RNA polymerase and E
genes4 was
used to detect the presence of SARS-CoV-2 .
Samples were collected on five days over a two
week period. One patient’s room was sampled
before routine cleaning and two patients’ rooms
after routine cleaning. Twice-daily cleaning of
high-touch areas was done using 5000 ppm of
sodium dichloroisocyanurate. The floor was
cleaned daily using 1000 ppm of sodium
dichloroisocyanurate.
Clinical data (symptoms, day of illness, and
RT-PCR results) and timing of cleaning were
collected and correlated with sampling results.
Percentage positivity was calculated for rooms
with positive environmental swabs. Institutional
review board approval and written informed
consent were obtained as part of a larger
multicenter study.
Patient A’s room was sampled on days 4 and 10 of
illness while the patient was still symptomatic,
after routine cleaning. All samples were
negative. Patient B was symptomatic on day 8 and
asymptomatic on day 11 of illness; samples taken
on these two days after routine cleaning were
negative
Patient C, whose samples were collected before
routine cleaning, had positive results, with 13
(87%) of 15 room sites (including air outlet
fans) and 3 (60%) of 5 toilet sites (toilet
bowl, sink, and door handle) returning positive
results Anteroom and corridor samples were
negative. Patient C had upper respiratory tract
involvement with no pneumonia and had 2 positive
stool samples for SARS-CoV-2 on RT-PCR despite
not having diarrhea.
Patient C had greater viral shedding, with a
cycle threshold value of 25.69 in nasopharyngeal
samples compared with 31.31 and 35.33 in
patients A and B
Only one PPE swab, from the surface of a shoe
front, was positive. All other PPE swabs were
negative. All air samples were negative.
There was extensive environmental contamination
by one SARS-CoV-2 patient with mild upper
respiratory tract involvement. Toilet bowl and
sink samples were positive, suggesting that
viral shedding in stool5 could
be a potential route of transmission. Post
cleaning samples were negative, suggesting that
current decontamination measures are sufficient.
Air samples were negative despite the extent of
environmental contamination. Swabs taken from
the air exhaust outlets tested positive,
suggesting that small virus-laden droplets may
be displaced by airflows and deposited on
equipment such as vents. The positive PPE sample
was unsurprising because shoe covers are not
part of PPE recommendations. The risk of
transmission from contaminated footwear is
likely low, as evidenced by negative results in
the anteroom and clean corridor.
This study has several limitations. First, viral
culture was not done to demonstrate viability.
Second, due to operational limitations during an
outbreak, methodology was inconsistent and
sample size was small. Third, the volume of air
sampled represents only a small fraction of
total volume, and air exchanges in the room
would have diluted the presence of SARS-CoV-2 in
the air. Further studies are required to confirm
these preliminary results.
Significant environmental contamination by
patients with SARS-CoV-2 through respiratory
droplets and fecal shedding suggests the
environment as a potential medium of
transmission and supports the need for strict
adherence to environmental and hand hygiene.
Published Online: March
4, 2020. doi:10.1001/jama.2020.3227
STARC Systems to Provide Critical Isolation
Rooms
STARC Systems, a manufacturer of temporary
modular wall containment systems used for
occupied renovations, has been deemed
“essential” and will refocus all production on
instant isolation solutions to dramatically
increase the number of units available for
healthcare facilities throughout the country,
helping to protect more healthcare workers and
patients and reduce the spread of COVID-19.

Typically, STARC
Systems modular
wall solutions are used in occupied healthcare
renovations to eliminate dust, debris, and
pathogens from impacting patients and employees.
Now, rather than keeping pathogens from escaping
a construction site, these panels are used to
eliminate the spread of COVID-19 by creating
instant negative pressure isolation anterooms
and airborne infection isolation rooms (AIIR).
“For years we have relied on STARC Systems
solutions to provide a safe environment for
healthcare workers and patients during health
healthcare facility renovations,” said Brian
Hamilton, Director of Healthcare and Life
Sciences, Consigli Construction. “Now, STARC has
become critical in our response to provide
hospitals with immediate patient isolation rooms
to reduce the spread of coronavirus. These rooms
allow overflowing healthcare systems or entirely
repurposed facilities to separate patients who
are sick from other patients and healthcare
workers who are not.”
STARC Systems’ isolation rooms exceed the ICRA
Class IV and ASTM E-84 healthcare requirements
for infection control and fire/smoke spread and
its surfaces are easily disinfected. They have
continued to be used at national healthcare
facilities, such as Massachusetts General
Hospital, Cleveland Clinic, and Seattle
Children’s in occupied renovations to meet the
highest infection control standards.
Portafab Supplies Temporary Isolation Rooms
Whether to create a standalone airborne
infection isolation rooms with anterooms or wall
temporarily quarantine entire sections or floors
of a hospital, PortaFab offers solutions for
clean, quick construction.
-
Hospital Isolation Walls
-
Patient Rooms
-
Nurses Stations
-
Anterooms
-
Hospital Testing Facilities
-
Triage Centers
-
Temporary Hospitals in
Convention Centers
-
Deployable Shelters
-
Multi-Purpose Facilities

Healthmark Provides Tags, Labels, Trolleys for
Reprocessing N95 Masks but also Cooling
Accessories
Healthmark provides
ways to beat the heat within the SPD or
Operating Room with
lightweight, latex free cooling devices.
Simply submerge the head gear or neck band in
cold water for 2-3 minutes, and the enjoy hours
of cooler temperatures. For additional heat
relief, pair in one of the Cool Aids vests,
which utilize cold packs core body cooling.
Healthmark provides custom labeling solutions to
fit a variety of customers’ needs. As facilities
have started to reprocess N-95 masks, they’re
looking for ways to clearly identify those that
have gone through the decontamination process.
One of the custom labels created for a customer
is a small 5/6” x 1 ¼” green “CLEAN” label with
removeable adhesive that can put on masks after
reprocessing and removed prior to reuse.
Due to supply shortages, many facilities are
reprocessing N-95 masks after use. In order to
transport the high volume of these masks, a
customer is using
2220
trays and trolleys, traditionally designed for
flexible endoscope transport, to transport them
between the point of use and the Sterile
Processing Department.
With UV disinfecting systems being used for new
purposes and used more frequently, customers are
finding the UV Indicators (UVI-001) a useful
tool to verify the use of such systems.
Ortner Furnishes Fan Filter Units for Retail
Check Out
Ortner Cleanroom Unlimited” has been dealing
with comprehensive cleanroom technology for more
than 30 years and across all industries: from
pharmaceutical, medical, BSL (“Bio Safety
Level”) high-security laboratories and food
production to the mechatronics, electronics, and
microelectronics sectors. For around 20 years
now, Ortner Reinraumtechnik GmbH has specialized
in the development and manufacture of equipment,
systems, and processes for creating
microbiological and particulate cleanliness.
This is now applied to mitigating
coronavirus in many applications including
retail checkout
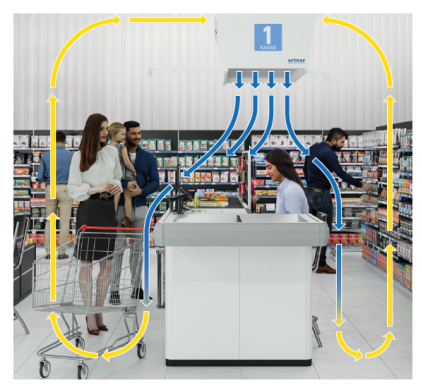
The system draws in the room air at the top and
blows the filtered particle- and germ-free air
in a quiet, low-turbulence piston flow over the
individual and the checkout zone.
The aseptic air flows out over the employee at a
flow rate of approx. 0.2m/s and envelops the
employee in a protective cover. This current is
usually not perceptible to humans, does not
usually cause draughts, and is established as an
international state-of-the-art technology.
The low-turbulence laminar flow flows freely
over the employee into the adjacent room and
thus suppresses possible contamination. The air
is transported from the ground back to the
suction point of the laminar flow system before
being filtered and reinjected in recirculation
mode.
Due to the high air volume (per system approx.
600 –1000 m³/h) and the permanent filtering to
100 % sterility, the entire room is extensively
cleaned. This creates additional protection in
the entire environment. The laminar flow systems
can be designed as pure air modules or with
options such as LED lighting (open green /
closed red), freely selectable coatings, design
elements, or advertising lettering.
The laminar flow system also offers protection
in other times of risk, such as for flu and
influenza, pollen allergies, or other contagion
hazards. The described effects continuously
clean the room air of fine dust and pathogens.
https://www.ortner-group.com/en/latest-information.php
Ortner Furnishes Three Way Airlock H2O2
Decontamination System
The Kantonsapotheke Zürich is one of the leading
hospital pharmaceutical competence centres in
Switzerland. The focus is on the use of the
latest technologies for the production of
remedies. For the new building at the
Kantonsapotheke Zürich a 3-way H2O2
decontamination airlock with integrated laminar
flow was developed by Ortner Reinraumtechnik.
H2O2 airlocks are among the most practical
decontamination systems in the life science and
pharmaceutical sectors. The environmentally
friendly H2O2 technology allows an almost
unlimited application for material-friendly lock
processes. Based on past experience and
innovative plant engineering, H2O2 lock
technology is a very safe decontamination method
and is "state of the art" today. In addition to
perfecting H2O2 input and output, Ortner has
been working in recent years on the development
of high-performance compact systems. Thanks to
this system development, safe and flexible H2O2
solutions are available for a wide range of
applications.
The special feature of the installed H2O2
decontamination airlock was to construct a 3-way
airlock in order to safely guarantee the loading
situations for all 3 infeed and outfeed
directions. The challenge here was, despite the
3-way solution, to ensure the best possible
distribution of the hydrogen peroxide via
additional ceiling nozzles.
In order to be able to reproduce the complex
production process easily for daily use, a
comprehensive decontamination concept was
developed and introduced. Together with the
Kantonsapotheke Zurich, the monitoring system
was developed and integrated to meet the
specific challenges of the pharmacy. The user
can now select different loader recipes for
different loading conditions. A validated system
was handed over.
For the Cantonal Pharmacy of Zurich it was a
great challenge to define the exact loading
situations in advance. Because the fact is:
Optimized cycle development and validation is
only possible if the batch carts are always
consistently loaded. In this project, Ortner was
able to achieve a fast cycle of approximately 30
minutes for the loading conditions glass and
metal containers. For the plastics (overpacked
Petri dishes, overpacked disinfectant
containers, PE bags, etc.) a cycle time of less
than 60 minutes could be achieved through
optimal distribution logistics on the loading
carts. The total cycle time is mainly influenced
by the absorption behavior of the objects,
especially of plastics in the airlock. This
means that the most critical time factor is in
the aeration phase, as the airlock can only be
unloaded again when the prescribed mak value of
< 0.5 ppm is reached.
Various materials show a strong absorption of
airborne hydrogen peroxide during the
decontamination phase.
This is followed by a long desorption phase
during the ventilation of controlled
environmental conditions such as an H2O2
airlock. A clumsy choice of material or an
incorrect loading configuration can therefore
possibly lead to a very long ventilation phase.
This phenomenon is one of the main reasons for
the discrepancy between theoretical and real
values when estimating the aeration phase of a
plant to be decontaminated with hydrogen
peroxide. For this reason, our design of
decontamination plants is based on a special
simulation program, by which e.g. the placement
of the injection areas for hydrogen peroxide can
be defined exactly in advance. Thus, a very
precise indication of the expected cycle times
can be realized.
As with any decontamination project, in the
final phase a cycle validation and all necessary
measurements using chemical and biological
indicators were performed to provide evidence of
a log-6 reduction. Sterility testing as well as
weld seam testing and weld seam documentation
were part of the qualification in order to meet
the GMP (Good Manufacturing Practice)
requirements.
To determine the "decontamination value", i.e.
the time period during which a biological
indicator is inactivated in a quantum region
based on a series of experimental data, Ortner
uses the "Limited-Holcomb-Spearman-Karber
method".
To carry out this procedure, an exposure time
must be evaluated in advance. This is divided
into several time ranges of equal size, whereby
all three time ranges of the survival curve of
the biological indicator must be covered:
- The survival time (all survive);
- The Survival-Kill Window (mixed);
- The kill time.
Ortner
used this scientific method to verify the
bio-indicators used and to determine the D-value
for each sample location of a load. The biggest
advantage of this method is the reliable
determination of the shortest possible cycle
time, e.g. for H2O2-locks with high throughput
density.
Partitions Without Laminar Air Flow May Increase
Virus Transmission
Where social distancing isn’t possible,
temporary partitions help protect production
associates at Perdue Farms’ Milford, Del.,
facility.
This could actually increase the
transmission rate.

It depends on the air flow patterns.
McIlvaine has a recorded video explaining
why partitioning has to be part of a laminar
flow pattern and not an obstruction to it.
Solutions such as Ortner has provided for
checkout counters can be applied to food
processing lines.
Octanorm Supplies Partitions for a Large London
Emergency Hospital
The new 4,000-bed Nightingale emergency hospital
in London was opened recently but it is hoped it
will not be needed as urgently as previously
thought because hospitals in the capital are
coping better with the coronavirus.
It had been thought that intensive care units in London would
be overflowing by this point, but political
sources said they had been told the capital’s
hospitals were three-quarters full, which is
better than expected.
In a highly praised high-speed build involving
an unprecedented partnership between the NHS and
the Ministry of Defense, the vast ExCeL
conference centre in east London has been converted
into a hospital,
Ready To Take Up To 500 Patients In The First
Wave.
Telstar’s Micro Biosafety Cabinets are Suitable
for the Manipulation of Samples with Biological
Risk Level 3
The recent emergence of coronavirus 2019-nCoV in
Wuhan has led research centers to work in-depth
to find potential antivirals or vaccines to
treat or prevent novel coronavirus infection.
Given the rise in demand of the specific
technological equipment to address these
research processes, the company has reinforced
the production of Bio II Advance Plus and
BiOptima, the new generation of Class II
Microbiological Safety Cabinets (MSC) designed
for use in high-risk microbiological research
and high toxicity applications in laboratories
and hospitals, to ensure the maximum level of
operator protection.
The coronavirus nCoV2019 is a specimen
identified as a biological agent in risk group
3 therefore it must be handled in Class II type
A2 Biological Safety Cabinets within a safety
level 3 (BSL-3) containment laboratory
environment. Biosafety Level 3 pathogenic agents
are those microorganisms that can cause serious
illness in humans and as such constitute a grave
danger to personnel, with a high possibility of
transfer to other people who, nevertheless,
responds positively to an effective treatment or
prophylaxis.
The Telstar Class II Microbiological Safety
Cabinets intended for the manipulation of
microorganisms with biological risk levels 2 and
3, the Bio II Advance Plus and BiOptima, offer
such high-efficiency biological protection that
prevents direct contact between the sample and
the operator. They have been designed in
accordance with the European EN 12469 standard
for Microbiological Safety Cabinets and
certified by TUV Nord in Hamburg (Germany).
BiOptima provides operator protection with the
inflow air barrier, product protection thanks to
HEPA-filtered laminar downflow in the working
area and environmental protection through the
exhaust HEPA filter. Furthermore, the compact
Bio II Advance Plus has been designed to offer
the highest level of safety for the product, the
operator and the environment. With maximum
containment capability it always guarantees to
provide maximum safety.
All these series not only conform to the EN
12469 standard on Class II biological safety
cabins (30% exhaust, 70% recirculation unit),
but also comply to the main requirements
assigned by NSF / ANSI 49 (Class II A2), JIS K
3800, SFDA YY-0569 and AS 2252.
Telstar also specializes in the development of
integrated solutions for biological containment
areas (P3) and mobile biosafety units.
