
Coronavirus Technology Solutions
April 21, 2020
Viroblock Effective Against Coronavirus
Sciessent Antimicrobial in Nextera Mask
Spectrashield Meets Efficiency Requirements
Berry Global has New Material for Surgical Masks
NC State Develops Two Polymer
Spunbond for Masks Which Can Be Sewn and is
Washable
Sandler Building a New Non
Wovens Line for Mask Media
Mitsui Chemicals has Expanded Melt Blown
Capacity
Three Layer Wipe is a Good Solution for
Coronavirus Mitigation
Large Non-Woven Demand to Mitigate Virus
______________________________________________________________________________
HeiQ Viroblock NPJ03, an antiviral and
antimicrobial textile treatment was tested
effective against coronavirus.
Since its inception 15 years ago, HeiQ has
forged a solid innovation track record helping
brands improve textile products. Catalyzed to
action by the global fight against Coronavirus,
HeiQ launched HeiQ Viroblock NPJ03, an antiviral
and antimicrobial textile treatment which is
proven effective against human coronavirus
(229E) in face mask testing, significantly
enhancing the antiviral log reduction from 2.90
of untreated face masks to 4.48, over 99.99%
reduction of virus infectivity. A log reduction
of two is equivalent to 100 times the
effectiveness.
“Virologist Dr. Thierry Pelet of HeiQ’s
Scientific Advisory Board brought us a profound
depth of knowledge and accelerated our efforts
to address the urgent problem of a global
pandemic,” says Carlo Centonze, HeiQ Group
CEO. “Our goal is to prevent textiles from
becoming a host surface for propagating harmful
viruses and bacteria and contribute to reduce
the risk and speed of contamination and
transmission.”
Chinese protective masks producer Suzhou Bolisi is
the lead adopter of HeiQ Viroblock NPJ03.
Treated masks will be available on the market as
early as this month. American legwear
manufacturer Kayser-Roth is planning to add the
technology to their new product, Ghluv hands
protector, while Lufeng from China is evaluating
the technology on other types of fabric used for
garments.
“I’m impressed by HeiQ’s ability to fast track
such a complex innovation and bring this
breakthrough to globally critical products in
short time,” says Dr. Pelet of HeiQ’s Scientific
Advisory Board.
“HeiQ strives to help improve the lives of
people through bringing breakthrough innovations
to textiles. We have pushed hard to bring the
HeiQ Viroblock technology to reality to assist
at this time of urgent need for society around
the world,” adds Centonze.
HeiQ Viroblock NPJ03 is a unique combination of
vesicle and silver technologies designed to
inhibit the growth and persistence of bacteria
and viruses. The HeiQ vesicle technology targets
lipid-enveloped viruses, such as coronavirus,
providing rapid virus deactivation, while the
HeiQ silver technology inhibits the replication
of both bacteria and viruses. HeiQ Viroblock
NPJ03 can be applied to a wide spectrum of
textile surfaces including face masks, air
filters, medical gowns, curtains, drapes and
more. HeiQ also has a range of highly
wash-durable antimicrobial and odor control
textile technologies, called HeiQ Pure,
combining silver-based and bio-based materials
for all fabric types.
Both 229E and Covid-19 are two of seven types of
human coronaviruses. Besides testing on human
coronavirus (229E), HeiQ Viroblock NPJ03 also
demonstrates dramatically improved reduction of
virus infectivity against Influenza types H1N1,
H5N1, H7N9, and Respiratory Syncytial Virus
(RSV).
https://www.nonwovens-industry.com/issues/2020-04/view_features/face-masks-industry-responds-to-crisis/
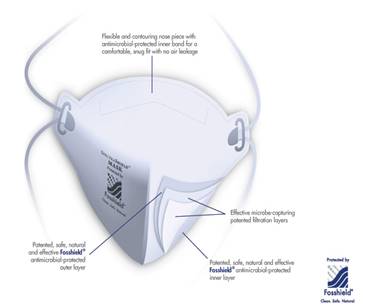
Sciessent Antimicrobial in Nextera Mask
Agion Antimicrobial (AM)
by Sciessent,
a
U.S.-based provider of antimicrobial (AM)
solutions based on naturally occurring elements,
is incorporated into FDA cleared N95 respirator
masks, the type most in-demand in the fight
against the COVID-19 pandemic. As of March 31,
2020, over three million Nexera SpectraShield
9500 masks incorporating the Agion AM have been
delivered to healthcare facilities worldwide.

The Nexera SpectraShield surgical respirator was
cleared by NIOSH and received an updated 510(k)
from the Food and Drug Administration in 2015
with approved claims to inactivate viruses by
99.99% in five minutes and kill 99.99% of
bacteria in one hour. It has also been cleared
in Canada and the European Union.
"Following the
viral outbreaks of the early 2000s, Sciessent
engaged with university researchers, industry
partners and government organizations to
investigate the ability of Agion to inactivate
viruses," says Paul Ford, CEO, Sciessent. "Once
Agion AM's anti-viral efficacy was proven,
Sciessent worked with Foss Performance
Materials to develop a polyester fiber, named
FossShield, with Agion AM embedded into the FPM
fiber itself. The FPM media was then
manufactured into N95 respirator masks sold by
Nexera Medical."
Spectrashield Meets Efficiency Requirements
The filtration technology in the SpectraShield™
Series of antimicrobial respirator masks
formally passed penetration and resistance in
multiple testing at numerous independent testing
laboratories in the European Union. These tests
require the SpectraShield™ masks to be subject
to exposure of a quantity of particulate
aerosols at .3 micron in size at a specific
velocity rate. Upon the exposure of the
aerosols, the amount of droplets that penetrate
the mask are measured. In the European Union,
for the masks to be rated a FFP2 it must meet a
minimum of a 97% filtration rate, and for a
FFP3, it must meet a minimum 99% filtration
rate.
In addition to conventional testing for a
disposable respirator mask, the SpectraShield™
mask was also subjected to rigorous testing for
reusability in which each mask was tested for
filtration performance, inhalation and
exhalation minimum tolerances after the masks
had been subjected to severe clogging in a
dolomite dust test. Two of the SpectraShield
masks passed this rigorous reusability testing
to earn a classification of FFP2 RD and FFP3 RD.
The tests
conducted at these various independent
laboratories were conducted according to
national and international testing standard for
filtration and safety. All the independent
testing laboratories used in testing the
SpectraShield™ masks are recognized by the
applicable regulatory agencies in the European
Union.
Extensive toxicology testing has been performed
by AgION regarding the silver-copper zeolite
antimicrobial agent. Independent tests results
indicate the antimicrobial agent to be safe and
non-toxic causing no negative side effects,
conditions, or consequences.
Berry Global has New Material for Surgical Masks
Berry Global will
increase production of face mask materials. The
initiatives include additional capacity for the
production of face mask materials in North
America and a new material for face masks in
Europe. With demand outpacing current supply for
face mask filter media, the product development
team at Berry has responded to deliver
innovative solutions in a matter of weeks to
support the demand. These solutions include
pivoting existing manufacturing assets and
creating alternative materials for face masks.
Berry has
expanded its proprietary Meltex platform to add
meltblown capacity in Waynesboro, VA. The line
will make meltblown materials which will
ultimately be used in surgical-grade face masks
along with N95 and N99 respirators. This added
capacity will support the manufacturing of
approximately 200 million face masks annually.
Berry is also
launching an extension to its Synergex range of
products, Synergex ONE, a new media for face
mask applications. Developed to initially meet
the new face mask categories for general
population, the aim is to quickly bring the
media up to EN 14683:2019 standards for
surgical masks. The newly introduced Synergex
ONE provides a multilayer nonwoven composite
product in a single sheet, as an alternative to
traditional face mask layer structures. This new
material will be manufactured in Europe and
serve the European market and is available
immediately.
“This was
something that was of paramount importance in
the short term development,” says Cedric Ballay
EVP & GM for Europe in Health, Hygiene, and
Specialties for Berry. “Given the array of
materials currently being offered to the market,
we are proud to offer an alternative solution to
the traditional charged meltblown. We are now
continuing to push on with the development to be
able to pass BFE Type I and Type II testing with
this media.”

Features are
·
Multi-layer composite material – no lamination
needed
·
Filtration core of unique meltblown technology
·
Suitable for general use
NC State Develops
Two Polymer Spunbond for Masks Which Can Be Sewn
and is Washable
NC State’s Nonwovens Institute (NWI) is using
its two research and training pilot production
lines to produce face mask materials that will
be used to protect medical workers on the front
lines of fighting the effects of COVID-19.
Surgical face masks are made with nonwoven
materials, says Behnam Pourdeyhimi, executive
director of NWI, Wilson College of Textiles
associate dean for industry research and
extension and William A. Klopman Distinguished
Professor.
Because of the current critical need for masks
caused by COVID-19, Pourdeyhimi and his NWI team
created a new spunbond material that can serve
as an effective filter without the need for a
meltblown filtration layer. The unique fabric is
composed of two different polymer materials that
are combined to make a single fiber with
significant strength and bulk – and that shows
effectiveness in filtration similar to current
materials used.
“Because of the COVID-19 crisis, we took the
spunbond technology and created a new generation
of unique filters that have excellent filtering
capability and can potentially be reused after
cleaning with peroxide, or potentially alcohol
solution,” Pourdeyhimi said. “Because these
materials are strong, unlike classical meltblown
filters, they can also be cut and sewn by
traditional techniques.”
Typically, one meter of spunbond material
provides enough material for about 20 to 25
masks when using the current designs,
Pourdeyhimi said. One of the NWI’s production
lines started producing 2,000 meters of spunbond
material per hour, with the potential to create
some 20,000 meters of spunbond material in a
day. NWI currently has an agreement to provide
large amounts of spunbond nonwoven material to
Brooks Brothers, which will make masks at its
manufacturing facilities.
NWI’s other production line is a
state-of-the-art meltblowing pilot line that
will make the classical meltblown material for
N95 masks and surgical masks.
“We created a recipe for the production of
classical N95 respirator materials and will ship
those materials out for industrial partners to
convert these into respirators,” Pourdeyhimi
said.
The meltblown material takes a bit more time to
produce; Pourdeyhimi estimates that his
production line can make about 12,000 meters of
material in one work shift.
Thanks to support from across the university,
Pourdeyhimi says that NC State has ordered
machines that will allow the NWI to make
surgical masks in its Centennial Campus
facilities. Those machines should arrive in the
next month.
https://news.ncsu.edu/2020/04/a-necessary-filter/
Sandler Building a
New Non Wovens Line for Mask Media
Sandler Group is investing in a high-tech
nonwovens line for the production of nonwovens
for respirator masks. Production is already
scheduled for the middle of the third quarter.
The new line will allow Sandler to produce
nonwovens for the manufacture of up to 800
million masks. The investment totals a
single-digit million Euro amount.
Mitsui Chemicals has Expanded Melt Blown
Capacity
Mitsui Chemicals has
expanded its production facilities for meltblown
nonwovens at wholly owned subsidiary Sunrex
Industry Co., Ltd., starting operations at the
new facilities in January. The move comes as an
effort to respond to growing demand for
industrial-use meltblown nonwovens and will
increase the Mitsui Chemicals Group’s overall
production capacity for these materials by 50%.
Mitsui Chemicals
is positioning its nonwovens business as a
growth sector, making efforts here to supply
high-quality nonwovens as industrial materials
for a variety of applications. This includes use
in car seats (product name: TAFNEL), masks
(product name: SYNTEX) and agricultural sheets
(product name: SYNTEX). With particular respect
to SYNTEX MB nano, marketing efforts are going
toward use in filters and other such
applications that will take advantage of the
meltblown nonwovens line’s superfine fibers,
which are no more than several hundred
nanometers in diameter.
Three Layer Wipe is a Good Solution for
Coronavirus Mitigation
FiberTect is
a three-layer, nonwoven wipe that features an
activated carbon core sandwiched between
absorbent top and bottom layers.
“It is widely used as the primary dry
decontamination method in hospitals and
ambulances,” says Corey Collings, a training
specialist for First Line Technology, which
markets FiberTect. “Hospitals use it in bulk and
in rolls, and ambulances use it in a kit called
the FastGrab to do immediate decontamination of
patients contaminated with a wide variety of
substances.”
FiberTect was invented by Seshadri
Ramkumar,
a professor of chemical countermeasures and
advanced materials in the Texas Tech Department
of Environmental Toxicology.
He says the wipe’s structure is effective in
containing bodily fluids – like saliva and mucus
– through which viruses could be transmitted.
Its activated carbon also can absorb particles
transmitted in vapor phase through the air.
As a wipe or mitt, FiberTect holds great
potential for cleaning in settings where
transmission of the COVID-19 virus, is a
paramount concern.
It can be used to clean wet surfaces
contaminated with bodily fluids,” Ramkumar says.
“Highly porous carbon in the structure can trap
the vapors and aerosols in which microbes are
contained. The wipe structure is flexible and
can take the shape of the objects to be cleaned.
The three-ply structure without glue helps this
effective cleaning.”
FiberTect has previously been used successfully
by the U.S. military to decontaminate both
personnel and equipment, for oil spill cleanup
during the Deepwater Horizon spill in the Gulf
of Mexico, and by emergency response teams
across the country in dealing with highly
dangerous chemical substances, including
Fentanyl.
Its development and testing was sponsored by the
U.S. Department of Homeland Security and managed
by the Technical Support Working Group, Office
of the Assistant Secretary of Defense for
Special Operations/Low Intensity Conflict in the
U.S. Department of Defense. Product testing was
conducted by Lawrence Livermore National
Laboratory. FiberTect proved superior in
all testing results against 30 comparable
products for decontaminating against toxic
chemicals.
Large Non-Woven Demand to Mitigate Virus
Phil Mango of Smithers says that three major
nonwoven based products can help with the
current coronavirus crisis. These are:
-
Face masks
-
Anti-bacterial (skin) wipes
-
Disinfecting (hard surface
wipes)
In addition, general purpose medical nonwovens
like underpads, gauze, and medical garments may
all be useful in treating any increase in
patients.
Meltblown capacity globally is only about
360,000 tonnes today; about 30,000 tonnes of
unused capacity was available prior to this
crisis.
If SMS (spunbond/meltblown/spunbond) is
included, though, the current production
capacity increases to almost 5.0 million tonnes
with almost 1.0 million tonnes of excess
capacity. Not all SMS would be suitable, though.
Already, significantly increased pricing and
demand for meltblown in some regions has been
reported. The key here may be converting
capacity, turning sufficient nonwoven supply
into face masks.
Anti-bacterial wipes are used for disinfecting
skin, usually hands. It appears that hand
sanitizing wipes containing at least 60%
alcohol should kill COVID 19 (it is an
enveloped virus which usually is susceptible to
these disinfectants); but only testing will
confirm that. Already, most producers have
maximized production in anticipation of
increased demand. These are based on spunlace
nonwovens, and globally spunlace has over
700,000 tonnes of excess capacity, much of it in
Asia. Producers are ramping up production as
consumer and institutional demand has increased
rapidly.
Hard surface disinfecting wipes are also being
recommended, even though it appears most viral
transmission is through person-to-person contact
via airborne fluids. Cleaning and disinfecting
wipes, based on standard and SP (spunbond/pulp)
spunlace with a quaternary ammonium
compound-based disinfectant solution, again are
expected to be effective against COVID
19. Already, producers are ramping up
production.
Most nonwoven producers can rapidly increase
production of needed products; converters may
have a more difficult time, as they tend to run
closer to full and have a more difficult time
changing from one product to
another. Additionally, disinfecting/sanitizing
solution and chemicals may be in short supply
initially.
In nonwovens, the only challenge may be
obtaining the correct raw materials and
balancing other product commitments. For
example, while there is ample supply of spunlace
for wipes, this is not universally true.
Spunlace supply in North America is tight, and
Europe is balanced. So, logistics may play
a role.
For converters, it is more difficult as
converting lines are typically dedicated to
certain products. For wipes, the challenge is
obtaining disinfectant solutions, and probably
re-arranging/adding packaging assets; quality
control resources may also be stretched.
For face mask converters, there may be an actual
shortage of converting capacity, which may be
impossible to address short term. Existing lines
will have to be run at maximum capacity and
efficiency.
The actual nonwovens, spunlaid and spunlace,
have more production and more available
production in Asia; but converting has better
availability in North America and Europe,
especially for wipes. Similarly, disinfecting
solutions that are specifically designed for
killing viruses have more availability currently
outside Asia, though this may not be as much an
issue.
China is already in crisis mode and nonwoven
producers, and converters are running at maximum
output already. Additionally, other regions are
already supplying Asia.
Face masks have been used in Asia for other
reasons at a much higher frequency than in other
global regions for many years, so supply lines
are established already and a new peak demand
for face masks should be best addressed
here. North America and Europe will be starting
from a much smaller base.
Wipes are just the opposite; North America has
the highest production rates and utilization
rates for disinfecting wipes and should have the
easiest time addressing new peak demands; Asia
will have the hardest time and may rely on
liquid disinfecting solutions and paper or
textile cloths.
