
Coronavirus Technology Solutions
April 14, 2020
Cummins and Dupont are Working Together to Help
Address the Current Shortage of N95 Masks
U.S Army Research on Face Mask Media will be
Helpful
People Should Not Walk/Run/Bike Close Behind
Each Other
FDA approves ASP H2O2 Decontamination Process
______________________________________________________________________________
Cummins and Dupont are Working Together to Help
Address the Current Shortage of N95 Masks
The need for masks has skyrocketed in recent
weeks due to the global pandemic, and Cummins
will use its NanoNet® Media to help answer that
need.
According to Amy Davis, Vice President of
Cummins Filtration, with many of the world’s
leading mask manufacturers in need of the
critical materials to assemble the masks
and struggling to meet demand, Cummins will
use pre-existing filter technology in
partnership with DuPont to help fill the supply
void.
"Cummins is re-evaluating our supply base and
manufacturing capabilities to identify how we
can support our healthcare professionals who
rely on critical personal protective equipment
to do their jobs," Davis said. "Our NanoNet®
Media can fill a key supply void and help
address the mask shortage facing the United
States and other countries around the world."
The project also aims to provide open source
instructions that other healthcare systems and
groups can use to create their own respirator
masks.
Cummins’ NanoNet® and NanoForce® Media
technology, which uses DuPont’s Hybrid Membrane
Technology (HMT), can typically be found in air,
fuel and lube filtration products used in
heavy-duty diesel engines to prevent long-term
engine wear, but also can be used in the N95
respirator masks worn by healthcare
professionals to filter harmful airborne
particles that can spread COVID-19.
The N95 designation means the respirator can
block at least 95 percent of particles from
entering the wearer’s nose and mouth. When
Cummins’ NanoNet® Media was tested using an
industry standard testing method, it exceeded
the performance requirements for N95
designation. Cummins’ manufacturing facilities
have since provided media samples to mask
manufacturers across the globe to test its
effectiveness.

Stock of Cummins Filtration NanoNet® and
NanoForce® Media technology winding.
While products featuring Cummins’ media will
need to be vetted and approved by the National
Institute for Occupational Safety and Health
(NIOSH), the company is preparing to do its part
to help relieve the burden facing the healthcare
industry.
“We’re working as quickly as possible with
healthcare regulators and other partners to help
certify products with our materials, and prepare
our manufacturing facilities to meet demand,”
added Davis.
The first mask prototypes using Cummins’ donated
media were assembled by University of Minnesota
teams in March as part of an initiative to
provide masks to M Health Fairview and other
Minneapolis-based healthcare systems. As the
COVID-19 outbreak escalated, the University of
Minnesota realized their supply of N95 masks to
protect healthcare workers would potentially run
out in a matter of weeks.
To address this challenge, a
team of designers, engineers,
chemists, surgeons,
anesthesiologist and apparel and
clothing experts from the
University of Minnesota’s
Institute for Engineering in
Medicine; Medical School;
College of Design; College of
Science and Engineering; and
Center for Filtration Research
Consortium (CFR) came together
to address this projected
shortage of critical personal
protective equipment.
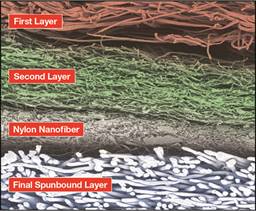
Advanced, high-performance media for N95
respirator manufacturing.
"The first thing we recognized from our experts
in the Center for Filtration Research, who work
directly with Cummins, is that not all
filtration materials are created equal and that
the Cummins material is an excellent
alternative," said Jakub Tolar, Campus Health
Officer and Medical School Dean at the
University of Minnesota.
"We are tremendously grateful for the generous
donation from Cummins of their filtration
materials toward our mask effort. Since the
arrival of the filtration media, we have been
able to make rapid progress, and we now believe
we have several viable mask options, including
both a disposable and re-usable option. These
designs show real promise in keeping our
healthcare workers safe should standard medical
supplies of N95 masks no longer be available,”
continued Tolar.
While DuPont’s innovative and unique Hybrid
Membrane Technology (HMT) is typically
integrated with Cummins’ synthetic fibers to
protect sensitive engine components, it has
multiple other applications that can include
filtration media used in N95 respirator masks.
DuPont’s Hybrid Membrane Technology goes beyond
the limits of traditional semi-porous or
nonwoven membranes for air and liquid
filtration. Made using a proprietary spinning
process, the hybrid technology materials are
comprised of continuous sub-micron fibers. The
end result is a “membrane-like” sheet structure
that balances breathability and high filtration
efficiency of particulates.
“We are proud to make our advanced technology
available to help protect more caregivers on the
front lines of this global health crisis,” said
HP Nanda, Global Vice President & General
Manager, DuPont Water Solutions.
“We thank our partner Cummins for transitioning
the use of its production line to help address
the global shortage of N95 mask materials, and
we thank the experts at the University of
Minnesota for their leadership in testing and
designing several mask options for the benefit
of many healthcare systems," Nanda added. "By
working together—and innovating new applications
of existing technologies and materials—we hope
to slow the spread of this terrible virus."
U.S Army Research on Face Mask Media will be
Helpful
The U.S. Army Edgewood Chemical Biological
Center (ECBC) Respiratory Protection Branch
members investigated novel aerosol filtration
materials for inclusion in the next generation
respirator. Commercial particulate filtration
technologies with high-efficiency and
low-pressure drop have the potential to provide
improved protection to the Warfighter while
decreasing breathing resistance and thus
reducing physiological burden.
A pressure drop of ≤5 mmH2O was selected as the
goal for the development of next generation
lower burden filters.
The aerosol filtration penetration requirement
for the M61 filter is ≤0.01% (i.e., 99.99%
efficiency) when measured at a constant flow
rate of 25 L/min (equivalent to 50 L/min through
the pair of filters). Each filter has an
effective airflow area of approximately 60 cm2 ,
which results in a face velocity of
approximately 7 cm/s when measured at 25 L/min.
The particulate filter element of the M61 filter
consists of pleated HEPA media and is roughly 6
mm thick. The market survey was limited to media
with the potential of achieving efficiencies
≥99.97% (HEPA quality).
While this target is below the JSGPM
requirement, efficiencies of 99.99% can be
achieved through pleating the media, which
reduces the face velocity and increases the
collection efficiency of the filter. This
reduction in face velocity increases the
collection efficiency of the filter. In the case
of flat sheet electrets (nonwoven electrostatic
charged media), the thickness can be increased
to meet HEPA requirements. Efficiency can be
improved by other means to maximize the
effective surface area, for example, by using
larger and more efficient filter designs similar
to those being considered for future integrated
respirator/helmet systems.
To avoid eliminating promising media, the market
survey did not take into consideration the
thickness of the media; however, a total
effective surface area of 250 cm2 was used as
the basis for the 5 mmH2O pressure-drop goal to
take into account the increased surface area
realized by the emerging advanced filter
designs. Taking these goals into consideration,
a market survey was conducted to identify new
HEPA quality filtration media with equivalent or
greater capture efficiency and lower pressure
drop than the particulate media currently used
in military air-purifying respirator filters.
Only commercial manufacturers were considered.
Here are the conclusions.
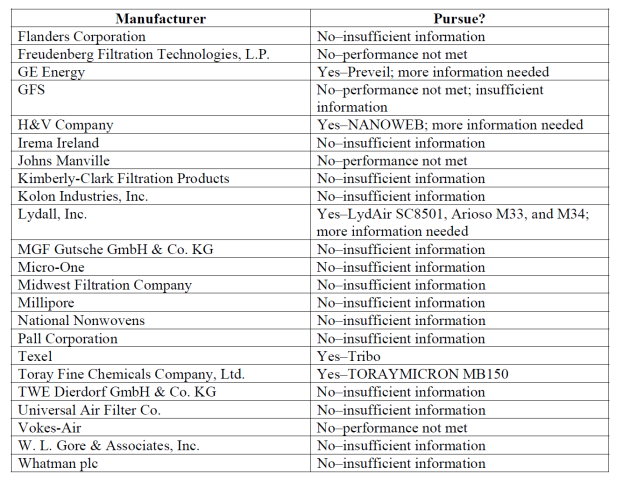
People Should Not Walk/Run/Bike Close Behind
Each Other.
As you can see from the depiction virus mists
can travel through the air from one runner to
another.
The typical social distancing rule which many
countries apply between 1–2 meters seems
effective when you are standing still inside or
even outside with low wind. But when you go for
a walk, run or bike ride you better be more
careful. When someone during a run breathes,
sneezes or coughs, those particles stay behind
in the air. The person running behind you in the
so-called slip-stream goes through this cloud of
droplets.
The researchers came to this conclusion by
simulating the occurrence of saliva particles of
persons during movement (walking and running)
and this from different positions (next to each
other, diagonally behind each other and directly
behind each other). Normally this type of
modelling is used to improve the performance
level of athletes as staying in each other
air-stream is very effective. But when looking
at COVID-19 the recommendation is to stay out of
the slipstream according to the research.
The results of the test are made visible in a
number of animations and visuals. The cloud of
droplets left behind by a person is clearly
visible. “People who sneeze or cough spread
droplets with a bigger force, but also people
who just breathe will leave particles behind”.
The red dots on the image represent the biggest
particles. These create the highest chance of
contamination but also fall down faster. “But
when running through that cloud they still can
land on your clothing” according to Professor
Bert Blocken.
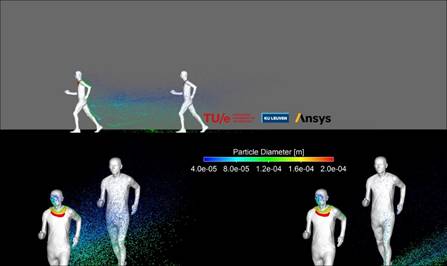
Out of the simulations, it appears that social
distancing plays less of a role for two people
in a low wind environment when running/walking
next to each other. The droplets land behind the
duo. When you are positioned diagonally behind
each other the risk is also smaller to catch the
droplets of the lead runner. The risk of
contamination is the biggest when people are
just behind each other, in each other’s
slipstream.
On the basis of these results the scientist
advises that for walking the distance of people
moving in the same direction in 1 line should be
at least 4–5 meter, for running and slow biking
it should be 10 meters and for hard biking at
least 20 meters. Also, when passing someone it
is advised to already be in different lane at a
considerable distance e.g. 20 meters for biking.
FDA approves ASP H2O2 Decontamination Process
With nearly 10,000 sterilization systems capable
of
processing 480 masks per day and the potential
for
three uses
ASP can be adding the equivalent of 480 x
3 x 10,000 = 1.5 million masks per day for use
by U.S. hospitals.
The U.S. Food and Drug Administration (FDA) has
provided an emergency
use authorization (EUA) for
a decontamination process provided
Advanced Sterilization Products (ASP)
that could see as many as 4 million N95
respirators per day sterilized for re-use.
That’s a significant potential dent in the
ongoing shortage of supplies faced by medical
professionals and frontline workers at
healthcare facilities.
This decontamination process would open up
re-use of N95 masks originally designed for
single use, and it uses vaporized hydrogen
peroxide gas to clean the respirators. ASP’s
STERRAD series sterilization machines, which are
covered under the EUA, are in use in around
6,300 hospitals already (they’re commonly used
for sterilizing other pieces of clinical
equipment but have not previously been intended
for use with N95 masks) and there are around
9,930 in operation across the U.S., each with
the capability of processing around 480 masks
per day.
The FDA has previously cleared another similar
system for N95 decontamination: Battelle’s
vaporized hydrogen peroxide process.
This new clearance greatly expands the reach and
potential volume of decontamination that’s
possible and should pave the way for others to
follow.
ASP
sterilization systems are used with many
hospital devices. Jeremy Yarwood, VP of research
and development says that all sterilization
systems and disinfectant solutions that ASP
provides have been tested against enveloped
viruses, the family of viruses that includes
coronavirus. Furthermore, some of our
products, including CIDEX® OPA,
have been directly tested against coronavirus,
and have been demonstrated to be efficacious.
