
High purity water needs in the power industry today are vastly different and much more varied than those of fifty years ago. In the United States there are still a number of fifty year old plants in operation. The classic design for water purification systems included clarification, low velocity or gravity sand filtration, carbon adsorption, two-bed deionization with hot caustic regeneration followed by mix-bed deionization. Today high pressure steam generators, concerns about chloride stress corrosion, chlorine pitting attach, colloidal silica carryover present operating problems that were not encountered with the older boilers. There are a variety of sophisticated schemes to produce high purity water.
Demands for higher purity water result from more efficient boiler designs. Coal-fired power generators have evolved to super critical designs. The nuclear power generation has its own unique demands. Requirements for high purity water are substantially reduced when using gas turbines. However, the combined cycle operation incorporating both a gas turbine and a steam turbine still requires approximately 10 percent of the high purity water required for steam boilers. Below is as diagram by Weir Plc of a coal-fired power plant.
Fossil Fuel Power Plant Pumps and Valves.pdf
Boiler Feedwater
Feedwater is
water added to a boiler to replace evaporation and blowdown. In many cases,
condensed steam returned to the boiler through the condensate system constitutes
much of the feedwater. Make-up is any water needed to supplement the returned
condensate. The make-up water is usually natural water, either in its raw state
or treated by some process before use. Feedwater composition therefore depends
on the quality of the make-up water and the amount of condensate returned.
Feedwater purity is a matter both of quantity of impurities and nature of impurities. Some impurities such as hardness, iron and silica, for example, are of more concern than sodium salts. Feedwater purity requirements depend on boiler pressure, design and application. Feedwater purity requirements can vary widely. Low pressure, firetube boilers require less stringent feedwater control than modern high pressure boilers.
Dissolved bicarbonates of calcium and magnesium break down under heat to give off carbon dioxide and form insoluble carbonates. These carbonates may precipitate directly on the boiler metal or form sludge in the boiler water that may deposit on boiler surfaces. Calcium sulfate, upon heating, becomes less soluble. Sulfate and silica generally precipitate directly on the boiler metal and ordinarily do not form sludge. For this reason they are much harder to condition and may cause more difficulties.
Silica is usually not present in very large quantities in water, but under certain conditions it can form an exceedingly hard scale. Suspended or dissolved iron coming in with the feedwater will also deposit on the boiler metal. Oil and other process contaminants can form deposits as well as promote deposition of other impurities. Sodium compounds do not deposit under normal circumstances. Sodium deposits can form under unusual circumstances: in a starved tube, a stable steam blanket or under existing porous deposits.
Chemical treatment of water inside the boiler is essential whether or not the water has been pretreated. Internal treatment, therefore, compliments external treatment by taking care of any impurities entering the boiler with the feedwater (hardness, oxygen, silica, iron) regardless of whether the quantity is large or small.
In some cases external treatment of the water supply is not necessary and the water can be treated by internal methods alone. Internal treatment can constitute the sole treatment when boilers operate at low pressure, much of the condensate is returned and the raw water is of good quality. However, in moderate and high pressure boilers, external pretreatment of the make-up water is mandatory for good results. With today's higher heat transfer rates, even a small deposit can cause tube failures or wasted fuel.
The purpose of an internal treatment program is fivefold:
To react with incoming feedwater hardness and prevent it from precipitating on the boiler metal as scale
To condition any suspended matter such as hardness sludge in the boiler and make it nonadherent to the boiler metal
To control the causes of boiler water carryover
To eliminate oxygen from the feedwater
To provide enough alkalinity to prevent boiler corrosion
In addition, a complete treatment program should prevent corrosion and scaling of the feedwater system and protect against corrosion in the steam-condensate systems.
Today's modern powerhouse uses a wide variety of internal treatment chemicals. Phosphates had been the main scale conditioning chemical until development of chelate and polymer type chemicals. Chelate programs offer superior cleanliness over phosphate programs, however, one weakness is the potential for corrosion if overfed.
All internal treatment, whether phosphate, chelate or polymer, condition the calcium and magnesium in the feedwater. Chelates and polymers form soluble complexes with the hardness, whereas phosphates precipitate the hardness.
Sludge conditioners (natural organic materials and synthetic polymers) aid in the conditioning of precipitated hardness. These materials must be effective and stable at boiler operating pressures. Certain synthetic organic materials are antifoam agents.
The demands on water treatment chemical companies are changing with the generation technology. R. Henry Weed of BetzDearborn compares the traditional boiler water treatment needs of a heat recovery steam generator (HRSG) operating at less than 1000 psi (6.89 Mpa) to the new treatment needs of the higher pressure boiler greater than 1501 psi (10.35 Mpa) seen recently.
One area frequently overlooked in new boiler system design is potential oxygen corrosion in the preboiler area. The combination of heated water and oxygen is very aggressive and action must be taken to minimize oxygen pitting corrosion. This problem has been seen in unlined "condensate" tanks and steel condensate" piping, which actually hold water that is a combination of hot condensate and fully oxygenated demineralized water. Options to prevent this type of problem include additional mechanical deaeration, revised metallurgy, and relocated chemical injection points.
Another problem area is the low-pressure (LP) drum of the HRSG. It is often the storage tank for the intermediate (IP) and high-pressure (HP) boiler feedwater pumps. Newer designs have even made this portion of the system a low-pressure steam generating section. This dual use creates two types of treatment problems.
First, since the LP drum provides HP and IP feedwater and attemperating water, no inorganic solids can be present in the water. This eliminates the possibility of using a coordinated pH/phosphate treatment program. Therefore, the boiler pH can only be maintained at the same level as the feedwater to the LP drum. Typically, feedwater pH is only kept in the 8.8 to 9.2 range for systems with copper metallurgy, and up to 9.6 in all-steel systems. These pH levels are lower than the range recommended to minimize any possible contribution to erosion corrosion in the LP tubes.
The second problem is trying to maintain the pH of the LP drum water. In an application with the LP drum producing steam, any highly volatile condensate treatment product will tend to volatilize and leave with the steam. The net effect of this loss of product is a decrease in the HP and IP feedwater pH. For this reason, neutralizing amines with lower distribution ratios, rather than ammonia, are preferred for feedwater treatment.
Condensate Polishing
Condensate polishing is a great concern in high pressure boilers
where condensate represents the bulk of boiler feedwater. Thus, it becomes the
major source of contaminant introduction. The condensate polishing process must
deal with impurities that are generated within the steam system itself rather
than those in the raw water. However, one of the sources is the impurities that
pass through the ion exchange systems used on the raw water side and then keep
recirculating and building up in the condensate system. condenser leakage is
another obvious source of dissolved contamination. Another source of dissolved
contaminant intrusion is air-in leakage. This will introduce carbon dioxide into
the cycle. CO2 reacts with pH conditioning chemicals to form stable
salts. While condensate polishing relies on ion-exchange technology, it is not
merely another form of demineralizaiton. There are major differences between
condensate polishing systems and ion-exchange systems used with the raw water
purification. Condensate polishing systems operate at high flow rates. They are
approximately one order of magnitude higher than raw water ion-exchange at 50
gpm/ft2 vs. 10 gpm/ft2 in general, the ideal properties
for a condensate polishing resin are good hydraulics, stability under thermal
and osmotic shock and mechanical stress, resistance to oxidation inorganic
fouling, high capacity for iron and crud, and effective filtration. As in
demineralization, resin separation during regeneration is necessary because
treatment of cation and anion resins differs substantially. Cross contamination
of resins during regeneration must be avoided, even more so than in the case of
make-up demineralizers.
General
Most boilers (aside from small residential boilers) require treatment of feed water taken from lakes, rivers, and wells to ensure efficient operation of the boiler and other system components including turbines and condensers. Water with significant amounts of contaminants can lead to corrosion, wear, and loss of heat-transfer efficiency that can add to overall fuel costs and other system inefficiencies.
Raw Water
Treatment: Raw make-up water from lakes, rivers, or wells used for boiler feed systems must be treated to de-mineralize the water and to remove dissolved and suspended solids and other contaminants before introduction into the boiler.
Raw water processing for boilers typically involves up to four sequential process steps, depending on the quality of the incoming water. The four most common steps are aeration, coagulation, filtration, and softening.
Figure 1. Raw Water Treatment Process for Boilers

Aeration involves the mixing of atmospheric air with the water to remove undesirable gasses including CO2 and hydrogen sulfide.
Coagulation involves the addition of chemicals such as filter alum to coagulate and precipitate coarse suspended solids.
Filtration removes the coarse suspended particles and precipitate products from the coagulation process.
Softening is a process that may take one or more forms to remove calcium, magnesium, silica, and silt. Softening processes may include lime-soda treatment, de-mineralization, and Reverse Osmosis (RO). Pre-screening is important in RO systems and nanofiltration (NF) systems to prevent premature fouling of the membranes with particulate matter or biological growth products.
The above processes largely define front-end water treatment for power boilers utilizing surface or well water for boiler makeup.
Supercritical boilers are power boilers operating at pressures above the supercritical pressure for water (approximately 3200 psi). Reasons for the trend among utilities to adopt supercritical boilers include: reduced fuel costs due to improved thermal efficiency; reduced CO2 emissions; reduced NOx emissions; improved efficiency at reduced loads; and proven technology at costs comparable to subcritical boiler technology.
As a result of the higher operating temperatures and pressures of this class of boiler, an additional filtration step is required in the steam-condensate loop of the boiler system to ensure that pure water is provided for boiler operation. This additional filtration step (known as condensate polishing) is additional to the treatment processes described above for treatment of raw make-up water.
Condensate Polishing
In the case of supercritical boilers, a condensate polisher is required in the steam-condensate loop to protect the boiler and associated system from damage due to contaminants that may be introduced in the condensing loop of the steam cycle. Supercritical boiler systems are susceptible to carryover solids because there is no steam drum, and any contaminants in the return-condensate will be directly introduced along with the steam into system equipment. A possible source of contamination of condensate (and hence of boiler feed water) comes from leakage of condenser cooling water into the condensate stream. An expanded discussion of condensate polishing is provided by the Dow Chemical Company at the attached link (condensate polishing.pdf).
The following illustration shows a simplified process diagram for a supercritical boiler.
Figure 2. Steam-Condensate Loop for Supercritical Boilers
![]()
In general, the following water treatment processes are recommended for subcritical and supercritical boilers.
|
Recommended Water Treatment for Subcritical and Supercritical Boilers |
||
|
Treatment Type |
Supercritical Boilers |
Subcritical Boilers |
|
Condensate Polisher |
Required |
Recommended |
|
All Volatile Treatment (AVT) |
Allowed |
Allowed |
|
Oxygenated Treatment (OT) |
Recommended |
Allowed with condensate polishing |
|
Phosphate Boiler Conditioning |
None |
Allowed |
|
Boiler Blowdown |
None |
Recommended to control boiler solids |
Boiler Feed-Water Systems
Hot Topic Webinar (Pumps for Power Plan)
A one and one half hour webinar with a power point presentation on boiler feed-water pumps prepared and presented by Ed Simmons, PE of the Shaw Power Group is available by entering the following hyperlinks in your web browser.
Power Point (only) Shaw Power Group
Complete Hot-Topic Webinar (You will need to enter the recording password: hth851) https://mcilvaine.webex.com/mcilvaine/servicenbrshared.php?action=playback&recordID=25945842&recordKey=5FB1191B1774FE266FF7CB1C50E2E9112D3FFE0AD9481C10C01C64ED2B7BEC1C
Overview of Boiler Feed-Water Systems
Feed water systems are an indispensible part of a modern steam-generating plant, and the boiler feed pump (BFP) is literally the heart of the system responsible for delivering water to the boiler under conditions of elevated temperature and pressure. Failures of the pump can result in reduced plant reliability and efficiency, premature equipment failure, and in downtime for the boiler and loss of process steam or electrical power generation.
Figure 1. Boiler Feed-Water System
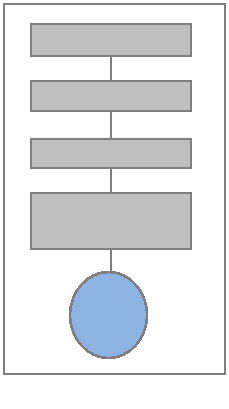
The de-aerator functions to remove gases (particularly oxygen) from the water in order to prevent corrosion within the boiler and other system equipment and piping. There are two major types of mechanical de-aerators: cascading tray-type de-aerators, and spray type de-aerators. De-aeration can also be achieved with the addition of chemicals, but in the power industry is typically accomplished using tray or spray systems.
Figure 2. Tray Type De-aerator (Illustration from Wikipedia)
The boiler feed pump (BFP) moves the water from the de-aerator tank toward the boiler and increases the delivery pressure to overcome the operating pressure within the boiler, which can be in the range of 2,400 psi for subcritical boilers, and in excess of 3,200 psi for supercritical and ultra-critical boilers. The modern practice of load-following in steam-generating systems to manage fuel costs has placed significant demands on boiler feed pumps to respond to variations in temperature and pressure, and even to start and stop operations. Modern feed water pumps, therefore, must be designed and built to reflect tolerance of changing operating conditions.
The feedwater heater preheats the water before introduction into the boiler to increase boiler thermodynamic efficiency and to prevent thermal shock. Typically, some portion of system steam from the high-pressure turbine is diverted to a heat exchanger to accomplish feedwater pre-heating.
Figure 3. Feed-Water Heater (Illustration from Wikipedia)
![]()
The regulation valve precisely balances the amount of water introduced into the boiler with the water leaving the boiler based upon boiler steaming rate. Correct modulation of feedwater into a boiler is critical to proper boiler operation.
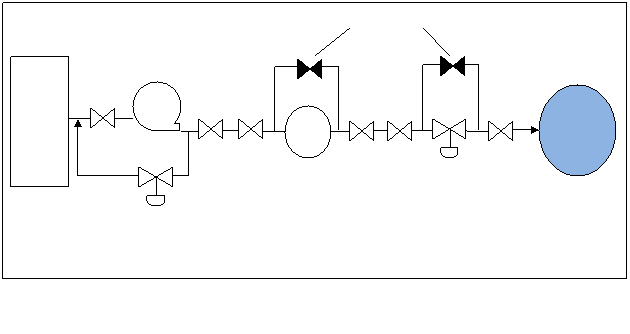 The
following illustration shows a simplified flow diagram with additional
re-circulation and bypass valving for a boiler feedwater system.
The
following illustration shows a simplified flow diagram with additional
re-circulation and bypass valving for a boiler feedwater system.
Figure 4. Feed-Water System Flow Diagram
For a more complete discussion of boiler feed pump systems, refer to the following industry tutorials and trade press articles.
http://www.indresinc.com/Assets/(IRI-07-SD)%20FeedwaterSystem.pdf
A boiler feed pump success story
Improving power plant reliability with boiler feed pumps
Flowserve Utility Barrel Pumps
Design
Steam from the boiler
passes through the turbine and expands most of its energy. To minimize the load
on the demineralizer and reduce operating costs, the residual low quality steam
coming out of the turbine is condensed in a heat exchanger, called a condenser,
and recycled to the boiler.
While this condensed water or "condensate" is much cleaner than the raw water entering the demineralizer, some corrosion products are picked up as the steam and condensed water passed through piping, heat exchangers, feedwater pumps, and other equipment in the steam turbine loop. A far more serious threat is the contamination that can occur if condenser cooling water with a high level of total dissolved solids (TDS), such as sea water, leaks into the condensate stream. Condensate polishing uses ion-exchange resins to remove contaminants from the condensate.
Ion-exchange resins can be used in a number of ways to treat condensate.
Cation Exchange - "Condensate Scavenging" is used mainly with industrial low-and medium-pressure boilers, a deep bed of a strong acid cation exchange resin operated in the sodium or amine form can act as "condensate scavenger." This type unit is primarily for the removal of corrosion products from the condensate. Insoluble particulate corrosion products are filtered in-depth on the resin bed and some hardness ions are interchanged with the cation on the resin. The choice of cation resin ionic form depends on the chemistry of the circulating water system.
The most common ion-exchange system used in condensate polishing is a mixed bed of strong acid cation exchange resin and strong base anion exchange resin. Mixed beds produce very high quality demineralized water, because ion leakage from either cation or anion resin is quickly removed from the water by the other resin. Deep-bed, in-depth filtration (Figure VII-12) is accomplished by maintaining the flow rate high enough to keep surface filter cakes from forming. Typically, the flow rate is about 50 gpm/ft.2. Using a bed depth of approximately 3 feet allows pressure drop across the bed to be maintained at economically acceptable levels. In most cases, a mixed bed condensate polishing system consists of several vessels operating in parallel (Figure VII-13). Used resins are transferred to a separate system for cleanup and regeneration. In some cases, systems employ disposable mixed bed resins.
External regeneration or regeneration of each resin outside of the condensate polishing vessel has proven to be the most practical approach. Isolation of the regenerant chemicals from the recirculating water loop significantly reduces the incidence of condensate contamination by regenerants. The amount of time that the polisher is off-line is reduced, as well. In external regeneration, the only interruption in polisher service is for transfer of the used resin to the regeneration system and the introduction of newly regenerated resin to the condensate vessel. One regeneration system can service multiple condensate polisher vessels. A typical external regeneration system is shown in.
This is the most widely used system today and requires these basic steps. The used resins must be:
Transferred completely from the operating vessel to the regeneration system.
Cleaned to remove the particulate contaminants collected by filtration from the condensate.
Separated as completely as possible for the regeneration.
Regenerated independently with the appropriate chemical solution.
Rinsed thoroughly with demineralized water.
Remixed carefully.
Transferred to the next available condensate polisher while exercising care to minimize resin separation.
When AVT is used to control pH and corrosion in a steam generator system, the condensate polisher in the system exhausts quite rapidly. This happens because the amine involved is exchanged onto the cation exchange resin. The higher the pH, the shorter the run time.
One suggested technique for increasing the run time on the mixed bed polisher is to treat the condensate with a hydrogen form cation resin to remove the amine prior to contact with the mixed bed. By taking this amine load off the mixed bed, mixed bed run lengths can be extended to months. Corrosion products are also removed by the lead cation bed, eliminating solids contamination of the mixed bed. Regeneration of the lead cation can be done on a more frequent basis than the mixed bed, thereby reducing the difficulties of mixed bed regeneration.
The use of the two beds in series, cation/mixed bed, increases the pressure loss across the system, increasing pumping costs. The development of ion-exchange resins with good bead size uniformity allows optimization of the pressure drop characteristics of the resins.
Cation-Anion-Cation Stacked Bed is a relatively recent development. This process uses a single tank with compartments to contain separate layers of cation, anion, and cation resins. The resins are never mixed, with each resin going to its own external regeneration vessel. The lead cation resin is typically not run past the ammonia break in AVT systems. Leakage from the lead cation is polished in the trailing cation resin. Final water quality produced depends on the trailing cation resin regenerant rinse-down, and on the leachable characteristics of both cation resins.
Cooling Tower Water
Cooling-water
quality, system operating parameters, and environmental restrictions have
greatly influenced the use of oxidizing biocides at utility power plants,
according to Paul Puckorius in Power Magazine. The most cost-effective of
these is chlorine gas, yet environmental concern and international disasters
have all but eliminated its use in the power industry today. While substitutes
have been applied to cooling-water
systems, regulatory restrictions make their continued use questionable. Thus, it
has become increasingly important to identify alternative biocides and
application techniques that can effectively control cooling-water
biofoulants and comply with permit requirements.
Water treatment for cooling systems now often incorporates sodium hypochlorite or bromine-producing biocides. Suspected toxicity, however, is bringing some bromine compounds into question. The US EPA is undertaking a study of chlorine in all forms, intended to ``prohibit, reduce, or substitute'' its use. Concerned with this trend, the Cooling Tower Institute (CTI), National Assn of Corrosion Engineers (NACE), and some industries have initiated a program to communicate with EPA, Congress, and other branches of government the need to keep chlorine available for cooling-water bio-control. Tightening restrictions, however, have prompted the search for chlorine substitutes as well as for entirely different approaches to biofouling protection.
Although utility cooling-water systems are basically similar, they vary sufficiently from plant to plant to warrant special biocide program considerations. Similarities include the use of alloy tubing in condensers and heat exchangers, linings in the condenser circuitry, and mild steel in waterboxes and some tubesheets. Design differences, however, have a strong impact on treatment needs and costs. Regulations that preclude the use of more effective chemicals necessitate a resort to less effective products that can entail significantly higher costs. Operating procedures often can be modified to take advantage of certain system design features--and the possible use of generic rather than proprietary chemicals--to reduce treatment costs.
Cooling systems at fossil-fueled plants differ substantially from those at nuclear stations, where biocide restrictions are even more stringent. Design and operating conditions at nuclear plants must enter into the selection of biocide programs to ensure effective biofouling control throughout the cooling-water system. Heading the list of factors controlling biocide choice is the source of cooling water. Raw water is the usual source. Consisting of untreated river or lake water, it typically contains ammonia, suspended solids, and organics, so a relatively high oxidant demand--5 to 10 ppm--usually is required to ensure an oxidant residual.
Systems are characterized by both a large capacity (typically 5-10-million gal) and long cycle time (capacity/circulation rate = 10 minutes or longer). These lead to considerable difficulty in maintaining the necessary oxidant treatment level. Use of film-pack fill instead of splash fill in cooling towers has led to excessive fouling with normal bio-treatment programs. This necessitates additional biocide attention to maintain cooling efficiency, possibly involving direct injection into the cooling tower. Other factors include water aeration, periodic condenser cleaning, etc.
Significant changes over the past decade include an increase in cycles of concentration (from an average of 3 to 5 to as much as 8 or 10) and use of scale inhibitors as a total or partial replacement for acid. As a result, pH levels have risen to 7.5 and higher--possibly to 9.0, where chlorine is much less effective. Presence of higher levels of organics and dissolved and suspended solids also have reduced the potency of biocide chemicals. Regulatory restrictions on plant effluent have introduced major limitations on oxidative biocides. Thus, a free-oxidant discharge limit of 0.2 ppm for two hours per day, common not too long ago, has in many cases been replaced by tighter residuals limits. To continue using halogens at levels high enough to maintain condenser cleanliness, utilities in some regions are now required to subject effluent water to de-halogenation to meet discharge regulations.
ClO2 can edge out bromine, particularly if both ammonia and high pH occur or in cooling water with high organic loading. Ozone is currently not in the running for utility plants larger than 100 MW or so, unless regulations prohibit use of chlorine and bromine compounds and capital cost is not a major factor. Non-oxidizing biocides only offer an alternative to chlorine in special cases.
| Discharge | Cooling | |||||||
| SIC Code | Title | No. of Facilities | High | Low | Average MGD | Total MGD | CFM | MGD |
| 4911 | Electric Services | 554 | 2670 | 0.01 | 500 | 277000 | 2000000000 | 360000 |
| 4930 | Combination Electric & Gas & Other Utility Services | 2 | 0.5 | 0.5 | 0.5 | 1 | 0 | |
| 4931 | Electric and Other Services Combined | 6 | 77.8 | 77.8 | 77.8 | 466.8 | 3067789 | 552.2 |
| 4939 | Combination Utilities, NEC | 1 | 868.1 | 868.1 | 868.1 | 868.1 | 0 | |
NOx reduction is accomplished in a number of combustion turbines through the injection of steam. For these installations large quantities of pure water are required. Unlike the typical coal-fired process scheme, the steam used for NOx reduction with gas turbines is a once through process. However, with the advent of dry low NOx burners, it is unlikely that there will be many gas turbines which utilize steam injection for NOx reduction installed in the future. Recently the size of combustion turbine plants has grown substantially and more sophisticated feedwater treatment systems are required. Also the control requirements are substantially greater than in conventional coal-fired systems. Feedwater control systems often require specialized designs to accommodate equipment limitations and the process dynamics which include integral deaerator and multiple pressure recovery steam generators. Under transient conditions the combustion turbine exhaust energy cannot be evenly absorbed in the high, intermediate and lower pressure evaporator surfaces of the heat recovery steam generator. To minimize the need for sophisticated feedwater controls, the mechanical design of the heat recovery steam generator and feedwater systems controls must account for the dynamic effects.
RO and other membrane techniques, are the focus of most of the utility activity in recent years designed to improve feedwater chemistry by enhancing the efficiency and reliability of makeup demineralizers and reducing the cost of their operation. Scaling is a primary concern because the RO reject becomes more concentrated as it passes along the membrane surfaces. Pretreatment to prevent scaling may include continuous feed of an anti-scalant and/or sulfuric acid. Sometimes sodium softening ahead of the RO may be needed.
Power producers are well aware of the economic penalties they incur when a component failure causes a plant shutdown. One of the heaviest financial burdens is attributed to steam-cycle corrosion, which is said to account for about half of the forced outages experienced in the US electric-utility sector and about $3-billion annually in operating and maintenance costs. Lowered costs are possible by improving cycle chemistry, because of the high benefit-to-cost ratios obtainable--in some cases as high as 1000:1. Upgrading chemistry monitoring with a continuous sodium analyzer at a cost of a few thousand dollars is a classic example. Keeping track of a steady increase in that feedwater contaminant, with its potential for turbine and superheater caustic corrosion if unchecked, can eliminate millions of dollars in maintenance costs.
As combined-cycle plants provide increasing amounts of generation, operators learn that heat-recovery steam generators must be handled differently than conventional boilers. In the past, heat-recovery steam generators (HRSGs) primarily served lower pressure industrial applications to recover heat from chemical or manufacturing processes. They operated at around 500 psig and used softened water as boiler-water makeup.
Today, HRSGs operate at substantially higher pressures and in a variety of complex configurations. They use sophisticated water treatment systems--including reverse-osmosis units, demineralizers, and condensate polishers--and play an integral role in the combined-cycle power plant that dominates new capacity construction.
The multi-drum configuration of HRSGs can make chemical cleaning difficult without additional piping and fittings. As a result, plants sometimes skip the pre-commissioning cleaning altogether. This leaves mill scale that provides preferential initiation sites for deposits and under-deposit corrosion. The longer this corrosion goes untreated, the worse it gets.
In many instances, HRSGs are over-treated. For example, vendors may recommend chelates or polymers to trap corrosion products and keep them in solution. This type of chemical treatment, which may work well on conventional industrial boilers, requires continuous blowdown, with a properly configured blowdown header to remove the corrosion products. In HRSGs, blowdown piping may not be designed with this in mind. Many times, blowdowns cascade from the h-p to the l-p drum. Therefore, any suspended corrosion products that escape the drum are not discharged from the system but are sent downstream where they can cause problems in a lower-pressure boiler. Also, the cycling nature of HRSGs may create sporadic periods of boiler blowdown, which is not conducive to removing suspended contaminants.
If a standard phosphate program is used, HRSG operators should lean toward the low-level phosphate program typical in utility boilers operating at higher pressures. Equilibrium phosphate treatment (EPT), all-volatile treatment (AVT), and oxygenated treatment (OT) programs have been used successfully in HRSGs. These programs add fewer chemicals into the boiler and feedwater, and put a stronger emphasis on ensuring high-quality makeup and condensate returns to produce pure feedwater.
Good control of O2 scavenger also is important for HRSGs, during startup as well as routine operation. Preventing corrosion and corrosion-product transport into an HRSG is a much better tactic than attempting to treat deposits once they settle in the unit.
The following case histories demonstrate water treatment in the power industry.
Case History 1 - A Major Gulf Coast Oil Refinery (Figure PO-4) 191 MW combined-cycle cogeneration facility, located on the Gulf Coast of Texas, began operation in 1986. With the Brazos River as the source, plant water is pretreated and demineralized to feed two 1,250 psig waste heat recovery units at up to a maximum flow of 2,500 gpm. River water pretreatment consists of chlorination, lime-softening clarification using a polymer and coagulant and followed by multi-media filtration. The pretreated river water is then roughly demineralized in an electrodialysis reversal membrane system with further demineralization by ion-exchange. The ion-exchange system consists of cation and anion-exchange resin beds, followed by mixed-bed polishing.
Case History 2: Gas and Oil-Utility - FPL's Turkey Point Fossil Station (Figure PO-6) is a two-unit 800 MW gas and oil-fired station, which has been in operation since 1967. Potable water is purchased from a nearby city for the plant's water supply. The makeup demineralizer is designed for 200 gpm capacity. The water is pretreated by multimedia filtration followed by acidification and forced-draft deaeration to reduce alkalinity and enhance overall water recovery. It is then demineralized by a multi-membrane system consisting of UF, RO and EDI. Final polishing is accomplished by off-site-regenerated mixed-bed resin polishers.
Case History 3: Gas and Oil - Utility - North Lake (Figure PO-7) is a three-unit, 734 MW gas and oil-fired station located in the Dallas-Fort Worth area in Coppell, Texas. Trinity River water pumped to the plant's cooling lake, is the source. The makeup demineralizer is designed for a maximum 150-gpm capacity. Lake water pretreatment consists of polymer and ferric sulfate-fed adsorption clarification followed by multimedia filtration.
Case History 4: Coal - Utility - Central Illinois Public Service Company, Coffeen Generating Station (Figure PO-9) is a 913 MW coal-fired generating station outside of St. Louis in operation since 1965. Lake Coffeen supplies the water to the makeup demineralizer system which is designed for a maximum 400 gpm capacity. The pretreatment system consists of a polymer-fed clarifier followed by media filtration.
Hyperbolic natural draft towers were at the leading edge of technology 10-20 years ago. Over 130 of these huge concrete monuments were built in the U.S. to copy the strong European trend. As of today there probably will never be any new hyperbolic towers built in the U.S. because the expected surge in the cost of energy/power to run the fans of a conventional mechanical draft tower never occurred. The hyperbolic towers cost over five times as much in front end capital cost as mechanical draft towers.
Conventional mechanical draft towers will be the technology used for the forseeable future. Most IPP's and Cogen plants going in new today will have towers because of thermal regulations for receiving waterways. More and more electric utilities are looking at recycling various waste streams, and often as tower makeup. This lowering of water quality in the tower system can force filtration consideration on makeup as well as on the tower water.
Some utilities such as American Electric Power have no filtration at any of their plants, whereas others retrofit their systems with filters when they have a problem, even though the water may have lower suspended solids than A.E.P. systems. This is the basic situation across the whole power industry. There is no clearly recognized limit of tower water suspended solids that cause problems of plugging. This is almost an individual preference like life insurance.
Perhaps 2-3 percent of all electric utility cooling water systems currently have side stream filtration today but with more and more plants forced to use lower quality makeup, and more restrictions on use of chlorine for disinfection, and with pressure to use lower cost high efficiency film pac plastic fill, there is a need for a lot more towers to use filters.