
Coronavirus Technology Solutions
June 10, 2020
Overview
June 10 Face Mask News
Membrane Mask with Silver Being Developed by UK
TTG has Membrane Media for Face Masks with a
Number of Claimed Advantages
Transparent Mask Developed by Swiss
Mask Policy Review
Should We Rethink our Whole Mask Policy?
Should task # 1 be to protect individuals rather
than keep them from being spreaders?
Virus Factors
Mask Factors
Wearer factors
Environment
Facemask Performance Articles in Previous Alerts
Silicon Based Membrane for Masks has Efficiency
and Other Advantages
Fibre Extrusion Technology Says There May Be
Better Answers Than Polypropylene for Meltblowns
NXTNano has Nanofiber Media Available for Face
Masks
Tustar Teams with Neatrition to Introduce High
Efficiency Masks to the U.S. Market
Sciessent
Antimicrobial Used in Hanesbrands Masks
Teho Filter Using Ahlstrom Media for N88 Masks
in Finland
Russian Scientists Develop Nanofibers for Masks
O2 Nano Mask is Low Pressure Drop, Efficient and
Reusable
Cambridge has an Efficient and Comfortable Mask
but with Valve
Masks are Part of a Combined Program to Reduce
the Odds to Near Zero
Airplanes are Relatively Safe with the Following
Guidelines
Midwest Textiles, Hollingsworth & Vose Partner
to Develop Homemade Facemask Kit
Bondex Ramps up Production of Material for PPE &
N95 Facemasks
Ultra-Pure PM 0.1 Mask Filter from Asiatic Fiber
Corporation
Ahlstrom -Munksio Media Being Used by French
Mask Makers
Ava Breathe has Unique Mask Design
High Fashion Mask is Available from Lumen
Couture
Website Dedicated to Analyzing Mask
Decontamination Options
N95 Masks Much Safer than Medical Masks for
Wearers
Avantor in Unique Position To Supply Products to
Mitigate the Coronavirus
Upscale KN95 Masks Available
Do we Need Masks with Valves for Certain People
and Certain Situations?
Chinese N95 Masks Shipped to Massachusetts did
not Meet Requirements
Alpha Pro Tech Expands Production of Face Masks
Masks with Valves have Advantages but Design and
Maintenance are a Challenge
ExxonMobil has New Filtration Fabric and Mask
Design
Spectrashield Meets Efficiency Requirements
Berry Global has New Material for Surgical Masks
NC State Develops Two Polymer Spunbond for Masks
Which Can Be Sewn and is Washable
Hospital Using MSA Respirators With Replaceable
Cartridge
MSA Has a Range of Respirator Designs Available
to Protect Hospital Personnel
Sindat Now Producing Efficient Masks with
Replaceable Membrane
Cummins and Dupont are Working Together to Help
Address the Current Shortage of N95 Masks
U.S Army Research on Face Mask Media will be
Helpful
Fibertex Non Wovens has New HEPA Filter Media
for Filters and Respirators
Alternative coverstock media from
Ahlstrom-Munskjo
Social Distancing Alternative with Low Risk and
Modest Cost for Coronavirus Mitigation
The Mask Market Could Soar From a Few Billion
Per Year to Hundreds of Billions
Superior Felt and Filtration has Both Meltblown
and Needle Punched Media for Masks
SWM supplies Melt Blown Media and Film for
Surface Layer
____________________________________________________________________________
Overview
Efficient masks remove virus aerosols and
protect wearers. Inefficient or loose fitting
masks may capture less than 20 percent of the
aerosols. New evidence reveals that virus
aerosols are a significant transmission route.
The guidelines and advice given to the public
are to wear masks not for self- protection but
to protect others. McIlvaine Company questions
this policy and is trying to understand all the
many factors which should impact mask
decisions. In this pursuit a webinar open to
everyone will be held on June 18 at 10:00AM
CDT. The proposition to be discussed and debated
is Should the primary use of masks be to
protect the wearer?
A number of recent research papers validate
airborne transmission of small virus aerosols
along with the possibility that even long
distance travelers may become dormant but are
revitalized when they are inhaled. Efficient N95
masks will capture these aerosols. However, this
type of mask is uncomfortable unless it is
equipped with a valve to allow unfiltered breath
to be exhaled. For this reason masks with valves
are prohibited in some communities and not
recommended in general guidelines.
Masks have proven to be extremely effective in
protecting asbestos workers, Asian citizens in
high air pollution areas and those with immune
deficiencies. The moon has a hostile atmosphere
but man has walked its surface. The common
denominator is that the danger of the conditions
is accepted and masks are designed to keep the
wearer safe. The webinar will probe ways that
more efficient N80 masks without valves can be
made available to billions of people. It will
cover the potential for a range of masks
including some with valves to be used depending
on individuals and circumstances.
This Alert has been expanded to include the
previous articles relevant to mask performance
and the various designs available and under
development. Important material selection
information is highlighted in red
Attendees are invited to submit data in advance
and participate in the general discussion during
the webinar. For more information and to
register Click
here
June 10 Face
Mask News
Membrane Mask with Silver Being Developed by UK
As the world continues to respond to the
COVID-19 pandemic, the University of Kentucky
continues its work to confront the virus through
innovative research and collaboration.
With funding and support
from Kentucky's National Science Foundation
(NSF)-sponsored Established Program to Stimulate
Competitive Research (EPSCoR),
a team from UK and Somerset Community College
(SCC) is creating 3D-printed, membrane-filtered
face masks that can inactivate the coronavirus.
The goal, through passive decontamination, is to
not only protect people from breathing in
viruses, but to eliminate them on contact.
Isabel Escobar, professor of chemical and
materials engineering in the UK College of
Engineering and associate director of UK’s
Center of Membrane Sciences, is working to
perfect the central component of the masks — the
filter. This filter will contain a unique
membrane composed of a polymer dissolved in a
nontoxic, bio-derived solvent, which will then
be chemically bound to medical-grade silver
nanoparticles, known for their antiviral
efficiency.
“The virus is about 120 nanometers in size — in
the world of membranes, that's large,” Escobar
said. “Even more so, it's not going to come as a
virus by itself, flying in the air. It's going
to come in the saliva, so it's going to be a
much larger particle. A large particle is just
not going through (this filter).”
But Escobar’s research takes it a step further,
adding the silver nanoparticles for the passive
disinfection.
“(The silver nanoparticles) prevent the virus
from binding and attaching, and it inactivates,”
she said.
Eric Wooldridge, professor of additive
manufacturing at SCC, and his team will be
providing the substrates, or the structure, of
the masks. These substrates, made of
polypropylene, will be 3D-printed in a honeycomb
pattern to allow for a strong, breathable
structure. The antiseptic membranes from
Escobar’s team will then line the insides,
ultimately creating a safer, cost-effective and
environmentally sustainable PPE that would match
or exceed N95 mask requirements.
“Utilizing SCC’s additive manufacturing
capabilities to produce the base components
combined with UK’s groundbreaking nanotechnology
to provide the coatings, our goal is to not only
demonstrate that it can be done, but that we can
rapidly scale production through our KCTCS
additive manufacturing network,” Wooldridge
said. “This collaboration represents one of the
primary goals of the KY NSF EPSCoR program:
bridging the gap between theoretical research
and practical application to rapidly respond to
a need and create solutions that truly matter to
the Commonwealth.”
“I love the fact that the 3D-printed supports
are from a collaboration with a community
college,” Esocbar said. “This shows that without
the help of a community college, this project
would not have been possible. We are, all
together, an intellectual force.”
Escobar, originally from Rio de Janiero, Brazil,
has spent the majority of her life working on
membrane filtration, with the focus of providing
clean water access to the world. When the
COVID-19 pandemic hit, Escobar realized her work
could also be applied to filtering viruses.
The team's antiviral masks have the potential to
positively impact the state, nation and world in
response to COVID-19. The collaboration is an
example of how KY NSF EPSCoR’s supported
researchers have the knowledge base and
creativity to pivot their research in the midst
of a public health crisis.
“KY NSF EPSCoR is in a unique position to see
connections between projects that are unknown to
researchers — without their vision, my idea of
making passive disinfection face masks using
membranes might have stayed as just an idea,”
Escobar said. “But thanks to the vision of KY
NSF EPSCoR, Eric Wooldridge and I connected and
were able to not only develop a short-term plan
to make face masks but also develop a long-term
plan for research at the forefront of
innovation.”
The KY NSF EPSCoR funding comes a month after a
similar project at UK was funded by an NSF Rapid
Response Research (RAPID) grant. This
project,
led by UK’s Dibakar Bhattacharyya (known to
friends and colleagues as “DB”), also seeks to
develop antiviral membrane masks, but through
different means. DB, who is director of the UK
Center of Membrane Sciences, is a mentor and
colleague of Escobar, and actually recruited and
brought her to UK in 2015.
“I have to say (DB) is probably one of the
people that I respect the most in the world,
probably one of the most brilliant minds alive,
and he and I spend a lot of time discussing
research ideas,” Escobar said. “We have a lot of
projects at the Center of Membrane Sciences. We
aim to determine how membrane science and
technology can help solve problems communities
are facing.”
http://uknow.uky.edu/research/uk-scc-team-confront-covid-19-antiviral-membrane-3d-printed-face-masks
TTG has Membrane Media for Face Masks with a
Number of Claimed Advantages
Trinity Technology Group’s (TTG) created a new
AIRADIGM three-layer composite medical face mask
media.
“We are proud that our proprietary AIRADIGM
technology can help better protect people on the
front lines of healthcare today,” said Greg Vas
Nunes, TTG’s CEO. “We applied our significant
experience in membrane and fabric construction
to create a composite material that provides
several unique performance features not
currently available.”
AIRADIGM composite face mask material is a
proprietary design that features:
·
Spun-lace polyester outside layer for
durability;
·
ePTFE membrane core for enhanced filtration and
breathability;
·
Spun-bond polypropylene inside for softness and
moisture management; and
·
The composite media is sonically laminated for
integrity and increased mask lifespan.
·
This unique, lightweight, bonded composite keeps
fluids and particles out but allows body heat
and vapor to escape for long term comfort. Also,
the composite’s membrane core is breathable and
increases the wearer’s protection. Studies have
shown that PPE comfort is a must for user
compliance and concentration, both of which are
absolutely critical in medical settings.
·
The proprietary membrane provides filtration
capabilities that do not degrade with humidity
unlike traditional melt blown mask materials.
Microbe and particle protection levels stay
consistently high for all-day protection without
frequent mask replacement. Masks made with the
special AIRADIGM media can be autoclaved and
safely reused. These benefits relieve the stress
on the PPE supply chain and improve return on
investment. Plus, substantially fewer masks end
up in landfills.
·
Third party testing and certification is under
way at Nelson Labs to confirm that the AIRADIGM
surgical grade face mask media exceeds ASTM
F2101 for Bacterial and Viral Penetration, ASTM
F1862 for Blood Penetration, and ASTM F2299 for
Particulate Infiltration, key criteria for N95
and ASTM Level 3 face masks.
Transparent Mask Developed by Swiss
A fully transparent surgical mask that filters
out germs but allows facial expressions to be
seen has been developed by Swiss scientists.
Caregivers should be able to wear them from the
summer of 2021.

For some segments of the population – like
children, the elderly and the hearing impaired –
the [current] masks are a major obstacle to
communication,” said the Swiss Federal
Laboratories for Materials Science and
Technology (EMPA) in a statement on Tuesday.
Along with researchers at the Swiss Federal
Institute of Technology Lausanne (EPFL), EMPA
has been working for two years on a completely
transparent surgical mask.
They have now finalized a biomass-based material
to manufacture the so-called HelloMasks and have
created a start-up called HMCARE, based on the
Biotech campus in Geneva, to market them.
The EMPA and EPFL researchers spent two years
finding the right combination of transparency,
resistance and porosity. They eventually came up
with a membrane made from a polymer developed
specifically for this application.
Because the new masks will be disposable for
optimal efficacy, like existing surgical masks,
the researchers focused from the start on
finding a material that was either recyclable or
biodegradable. “Our masks are made at 99% from a
biomass derivative, and we’ll keep working on
them until they’re completely eco-friendly,”
Pelet says.
Mask Policy Review
Should We Rethink our Whole Mask Policy?
Mask policy to combat COVID is based not on
protecting the wearer but on protecting others
from being infected by the wearer. There is an
argument to be made that the emphasis should be
the other way around.
If everyone is protected then stopping
the virus at its source is not so critical. It
is appealing to just focus on stopping the
source as you would by just turning off a garden
hose.
But if the water is already part of
a rain storm, source control is too
challenging. Virus in big cough droplets can be
likened to the garden hose whereas virus
aerosols can be likened to the rain storm.
We have mounting evidence that at least some
of the COVID transmission is through small
droplet aerosols or on virus attached to small
particles. The argument can be made that the
most important safeguard will be a highly
efficient N95 or even N99 mask. Here are some
examples
·
Medical personnel
are exposed to thousands of times more
COVID than others but they wear N95 or even more
efficient masks and avoid infection
·
60 choir members in Washington State attended a
2 hour choir practice but sanitized everything
and kept 6 feet apart. 45 of them became
infected.
If they had worn surgical masks maybe
only 25 would have become infected. If they had
all worn N95 masks none of them should have
become infected.
·
At a Southern China restaurant where the air
conditioner spread COVID N95 masks could have
prevented the spread.
·
If those passengers on the Diamond Princess
isolated in their cabins and inhaling viruses
through the HVAC system had instead roamed the
ship in N95 masks they would have been spared.
Should task # 1 be to protect individuals rather
than keep them from being spreaders?
What hospital would want to put efficient masks
on COVID patients but leave medical personnel
without protection?
In theory the concept works well. Just
control a few patients and then not have to
worry about anyone else. In practice the obvious
result would be disaster as COVID leaks would
soon fell the medical
workers. The takeaway is that it is very
difficult to prevent COVID from entering the
air.
The same could be said for the concept
that wearing inefficient masks will efficiently
capture any virus generated by the wearer Since
these masks are rated at very low efficiency on
0.3 micron particles and since they do not fit
tightly most aerosols will pass through or
around the mask
There are many arguments
to be made for and against this new
concept. They all depend on facts which are in
dispute or not clearly understood.
McIlvaine will be conducting a webinar on
June 18 to discuss all of the following
factors.
Virus Factors
·
The size and proliferation of aerosols
·
The percentage of virus in aerosols versus
larger droplets
·
The viral load
·
Minimum infectious dose
·
Life of virus
·
Virus rejuvenation from dormancy
·
Creation of aerosols from viruses leaving
surfaces
·
Efficiency of various masks in removing viruses
·
Various mask media options
·
Wash ability
·
Efficiency reduction over time or with washing
·
Mask fit
·
Comfort
·
Breathability and oxygen deprivation
·
Valve options
·
Killing as well as capturing viruses
·
Age and immune response
·
Other
medical conditions
·
Lung function
·
Activities
Environment
·
Virus load
·
Percentage of aerosols
·
Humidity
·
Air flow patterns
·
Benefits of capturing other contaminants
Virus Factors
Size and proliferation of Aerosols.
We breathe in millions of particles per minute
but must avoid just 10 viral particles.
Small particles such as virus aerosols are
invisible. This can provide a false sense of
safety. In every cubic meter of air we inhale we
also inhale 35 million particles greater than or
equal to 0.5 microns in diameter.
We inhale even more
smaller particles in the 0.1 to 0.2
micron range
which is the size of the virus.
The following table is designed to rate
cleanrooms.
ISO 14644-1 Cleanroom Standards
|
Class |
maximum
particles/m3 |
FED STD 209E |
|||||
|
>=0.1 µm |
>=0.2 µm |
>=0.3 µm |
>=0.5 µm |
>=1 µm |
>=5 µm |
||
|
ISO 1 |
10 |
2 |
|
|
|
|
|
|
ISO 2 |
100 |
24 |
10 |
4 |
|
|
|
|
ISO 3 |
1,000 |
237 |
102 |
35 |
8 |
|
Class 1 |
|
ISO 4 |
10,000 |
2,370 |
1,020 |
352 |
83 |
|
Class 10 |
|
ISO 5 |
100,000 |
23,700 |
10,200 |
3,520 |
832 |
29 |
Class 100 |
|
ISO 6 |
1,000,000 |
237,000 |
102,000 |
35,200 |
8,320 |
293 |
Class 1,000 |
|
ISO 7 |
|
|
|
352,000 |
83,200 |
2,930 |
Class 10,000 |
|
ISO 8 |
|
|
|
3,520,000 |
832,000 |
29,300 |
Class 100,000 |
|
ISO 9 |
|
|
|
35,200,000 |
8,320,000 |
293,000 |
Room Air |
There are some reports that the minimum
infectious dose for COVID -19 can be as low as
10 viral particles.
This means that if just a tiny fraction
of the particles we inhale every minute are
COVID we can become infected. For comparison
purposes a pharmaceutical cleanroom typically is
ISO 5.
The cleanest operating theaters in
hospitals are ISO 4. The semiconductor industry
spends billions of dollars per year to reach ISO
3. The task of keeping small particles such as
viruses from occupying space is very difficult.
Many of the particles we inhale are long
distance travelers. For example mercury emitted
from gold mines in Brazil has been traced to the
Artic. When a volcano erupted in Iceland the
skies turned dark in Europe for weeks. Italian
researchers have found COVOD on air pollution
particles in the Lombardy region. Another
takeaway is that social distancing has limited
effectiveness.
Viruses travel on cigarette smoke sized
particles.
So one way to view the task is to think
that everyone you encounter is puffing away and
you have to avoid even inhaling a few of his
smoke particles.

The percentage of virus in aerosols versus
larger droplets:
Viruses attach to droplets or particles.
They are only 0.1 microns in diameter but may be
in droplets 20 microns in diameter or larger.
Droplets in the 5 micron range can also be
generated or can be the result of evaporation of
larger droplets. In medical changing rooms in
China higher viral loads have been noted.
Viruses are also being aerosolized by cleaning
the floor or from other surfaces.
Viral Load:
The viral load varies by individual and
activity.
A lusty super spreader singer was able to
generate many thousands of aerosols and infect
45 people in just two hours.
Minimum Infectious Dose:
There are reports that only 10 viral particles
is enough to cause an infection.
Other views are that it generally
requires a large number of particles over a
period of time. Since large cough or sneeze
droplets don’t travel far, social distancing is
therefore the best way to avoid infection.
However, if small numbers of airborne viruses
can cause an infection then a whole different
approach is needed.
Life of Virus:
The virus is known to remain viable for hours in
the air and for days on various surfaces.
Virus Rejuvenation from Dormancy:
It has now been documented that viruses are not
necessarily dead but just dormant as they travel
through the air. They can then penetrate the
lungs where the moisture revives them.
Creation of Aerosols from Viruses Leaving
Surfaces:
There are numerous cases tracking aerosols which
were originally on surfaces such as floors or
clothing.
Mask Factors
Efficiency of Various Masks in Removing Viruses:
Masks vary in efficiency depending on the
media and the fit. Viruses average 120 nm
in diameter but can be entrained in droplets
larger than 300 nm. In general the efficiency
improves with more media which means higher
pressure drop. The meltblown media used with
most N95 masks is electrostatically charged
which improves capture efficiency.
The pressure drop impacts breathability. It
increases as the square of velocity. So where
there is lots of leakage such as the surgical
mask with the gap below, the actual pressure
drop is much lower than 2.5 Pa. this is because
much of the air is bypassing the media. It is
clear that surgical masks with gaps do not
remove a big percentage of small particles.
Most homemade masks are even more
inefficient.
In some cases a heavy cotton fabric is by
itself quite efficient but with high resistance
it will cause more air to bypass the mask. The
takeaway is that the masks being worn by the
public do not protect against virus aerosols.
Table 1. Filtration Efficiencies of Various Test
Specimens at a Flow Rate of 1.2 CFM and the
Corresponding Differential Pressure (ΔP) across
the Specimens
|
flow rate: 1.2 CFM |
|||
|
filter efficiency (%) |
pressure differential |
||
|
sample/fabric |
<300 nm average ± error |
>300 nm average ± error |
ΔP (Pa) |
|
N95 (no gap) |
85 ± 15 |
99.9 ± 0.1 |
2.2 |
|
N95 (with gap) |
34 ± 15 |
12 ± 3 |
2.2 |
|
surgical mask (no gap) |
76 ± 22 |
99.6 ± 0.1 |
2.5 |
|
surgical mask (with gap) |
50 ± 7 |
44 ± 3 |
2.5 |
The analogy can be made between a garden house
and a rainstorm.


If you only want to remove large cough droplets
it is as easy as dodging a garden hose wielded
by a baby.
If you want to stay dry in a major storm
that is very challenging.
Various mask media options:
Surgical masks are made mostly with
meltblown polypropylene.
The meltblown is sandwiched between two
layers of spun bond media. There is an
electrostatic effect which improves on the
already high efficiency due to the fine fiber
matrix. There are some new meltblown designs
with claims of even higher efficiency at a given
pressure drop.
There are a number of alternative materials
which are now available commercially. They
include nanofiber membranes which are claimed to
have higher efficiency at a given pressure drop.
They are also washable. The media is
available in large quantities.
In the case of Cummins the offering is
based on media originally designed for filtering
engine air intakes. In the case of Ahlstrom it
is the use of surgical drapes. Berry is another
supplier diverting media used from other
applications.
Efficiency:
Some of the newly available media has
efficiencies rated at N99 or better. 3M already
offers a range of options higher than N95 using
meltblowns.
The question of a carbon layer and its
impact on efficiency also needs to be addressed.
Wash ability:
N95 masks with meltblown media can be
decontaminated with UV light, H2O2 , or other
means. Battelle reports ten time successful
reuse of masks decontaminated with H2O2.
Masks made with membrane materials can be
washed by various means and reused many times.
This reuse ability results in a better
tight fitting mask with an affordable cost per
use.
Efficiency reduction over time or with washing:
The support structure as well as the mask media
can deteriorate. The ability to separate the
media and support structure means that the
structure can provide longer term use.
Mask Fit:
The mask fit is critical to providing
protection. The more expensive reusable masks
can provide that fit.
Comfort:
N95 masks without valves but with a tight fit
are uncomfortable to wear for long periods. The
medical worker can endure the discomfort for a
shift but it is unrealistic to think that
people will wear N95 tightfitting masks
without valves throughout the day without
serious negative impacts.
Breathability and oxygen deprivation:
With a tight fitting N95 mask the quantity of
air inhaled is down as much as 25%.
Some CO2 is re-entrained in the new
breath. Two Korean 14 year olds
who were required to run in 1000 meter
tests with their masks on died last month on the
track.
The government has since suspended the
requirement.
Valve options:
There is a large industry which has flourished
for many years. Masks with valves are purchased
by those wanting
to protect themselves from air
pollutants. In China there are Vogmask stores
where all they sell are dozens of varieties of
highly efficient designer masks.
They are equipped with one way valves
which allow the air to be exhaled through the
valve.
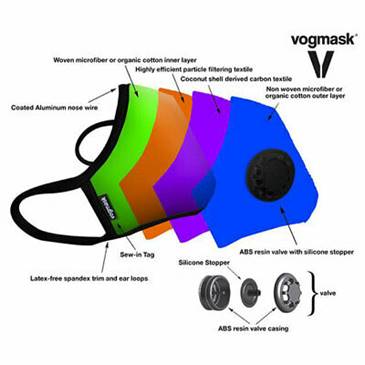
Millions of people have been wearing these valve
masks to protect themselves without concern
about their potential contamination of others.
Why is this not an important model for us to use
going forward?
Killing as well as capturing viruses:
A number of media designs incorporate silver or
other materials which will kill bacteria and
viruses.
Wearer factors
Age and immune response:
The use of masks by the elderly is conditioned
on the breathability.
It is unlikely that anyone with reduced
lung function or heart problems could wear an
N95 mask without a valve. Shouldn’t the type of
mask be dictated by this need? Should the
elderly be deprived of efficient masks because
valves are prohibited?
Sacramento says:
Masks with valves are prohibited.
Let’s consider mask recommendations by
segment and not as one.
Other medical conditions:
Those with impaired immune systems can probably
justify an N99 Mask and tight fit. This will
require use of a valve. The tight fit is also a
function of the valve. If you are blowing air
out around the edges of the mask you tend to
expand the opening.
Lung function:
The lung functions vary among individuals
creating an important variable in mask
selection.
Activities:
Any job function involving physical labor
including many meat processing jobs needs to
take into account the importance of wearing a
mask which does slow down oxygen intake.
Environment
Virus load:
The wearing of masks can be tailored to the
viral load and therefore the risk.
There is a movement to develop a N-80
mask which would be less efficient than the N95
but more efficient than the typical surgical
mask. Individuals could have all three mask
options available.
They could decide where it is most
appropriate to not wear a mask or to wear the
N80 or N95.
Percentage of aerosols:
The danger at any
point in time is a function of the
percentage of the total viral load which is in
the form of aerosols.
Humidity:
In general higher humidity deters the virus when
it arrives. The virus travels shorter distances
in humid air than in dry air where the droplet
size is smaller.
Air Flow Patterns:
The viral load is in part determined by air
flow. This important subject is dealt with in a
separate analysis.
Benefits of capturing other contaminants:
The coronavirus pandemic will subside at some
point in time. There will be uncertainty about
when and if it will return. Wearing an N90 mask
may become as much a habit as drinking bottled
water. Everyone is subjected to high air
pollution levels at some point in time during
the year. In some countries this is a daily
occurrence. But even in others the mask will be
justifiable. St Louis was meeting its
particulate ambient emission goals and was
assumed to be meeting its toxic metal goals
which had been shown to track the total
particulate. However, when the city installed
monitors to directly measure toxic metals, they
found spikes on days when the wind blew from the
direction of a lead refinery.
Hospital acquired infections cause 1.7 million
illnesses and 99,000 deaths per year in the U.S.
Patients would be well served to wear N95
masks
Face Mask Performance Articles in Previous
Alerts
Silicon Based Membrane for Masks has Efficiency
and Other Advantages
Researchers funded by King Abdullah University
of Science and Technology
have developed a membrane that can be attached
to a regular N95 mask and replaced when needed.
The filter has a smaller pore size than normal
N95 masks, potentially blocking more virus
particles.
N95 masks filter about 85% of particles smaller
than 300 nm, according to published research.
SARS-CoV-2 (the coronavirus that causes
COVID-19) is in the size range of 65–125 nm, so
some virus particles could slip through these
coverings. Also, because of shortages, many
health care workers have had to wear the same
N95 mask repeatedly, even though they are
intended for a single use. To help overcome
these problems, Muhammad Mustafa Hussain and
colleagues wanted to develop a membrane that
more efficiently filters particles the size of
SARS-CoV-2 and could be replaced on an N95 mask
after every use.
To make the membrane, the researchers first
developed a silicon-based, porous template using
lithography and chemical etching. They placed
the template over a polyimide film and used a
process called reactive ion etching to make
pores in the membrane, with sizes ranging from
5–55 nm. Then, they peeled off the membrane,
which could be attached to an N95 mask. To
ensure that the nanoporous membrane was
breathable, the researchers measured the airflow
rate through the pores. They found that for
pores tinier than 60 nm (in other words, smaller
than SARS-CoV-2), the pores needed to be placed
a maximum of 330 nm from each other to achieve
good breathability. The hydrophobic membrane
also cleans itself because droplets slide off
it, preventing the pores from getting clogged
with viruses and other particles.
https://pubs.acs.org/doi/full/10.1021/acsnano.0c03976
Fibre Extrusion Technology Says There May Be
Better Answers Than Polypropylene for Meltblowns
Fibre Extrusion Technology (FET), a UK-based
specialist in process solutions and equipment
for the manmade yarns and fiber extrusion
industry, has received unprecedented inquiries
about its nonwoven meltblowing systems since the
onset of the coronavirus crisis.
“We’re currently running trials, preparing
samples and defining specifications for
companies in Germany and Italy, as well as the
UK, and we could already have sold the lab line
we have here many times over,” said Managing
Director Richard Slack. “It’s primarily designed
for R&D and pilot scale applications, but trials
have proven it to be suitable for the low volume
production of critical meltblown face mask
materials. Some of the customers to whom we’ve
supplied similar lines have already pivoted
their production to this, which has generated
further interest.
“We feel, however, that we are ideally placed to
offer services to nonwoven companies who may be
exploring alternatives to polypropylene in
meltblown, due to our experience in working with
such a wide range of fiber types.”
FET’s meltblown system was originally developed
for companies looking to process high melt
viscosity medical grade resorbable polymers such
as polyglycolic acid (PGA), polylactic acid
(PLA) and polyhydroxl btyrate (PGH), mainly for
use in implantable products and other medical
devices.
The key applications for these fibers are in
hernia repair patches, staple reinforcement
buttresses, artificial skin, adhesion barriers
periodontal and ringival repair materials and
those for tendon and ligament repair.
“Our meltblowing system provides medical
companies and others dealing in such fibers with
a simpler processing route than other techniques
such as needle punching and a wide range of
structural and mechanical properties is
obtainable from batch production,” Slack said.
“There are also numerous options for
post-processing of the webs, by calendaring,
point bonding or lamination.”
Performance polymers such as TPU polyurethanes
and TPE thermoplastic elastomers are also
processed by a number of leading sportswear
companies on FET meltblown systems, while
engineering polymers such as ABS and PEEK, as
well as polycarbonate and halogenated polymers,
are other possible raw materials.
It is in the area of sustainable resins,
however, that FET believes much more can be
achieved.
Meltblown polypropylene nonwovens are the
critical component of the face masks needed for
Covid-19 frontline workers and their scarcity on
the open market has in part been the reason for
the reported shortages around the world.
An estimated 40 million face masks and other
disposable nonwoven-based PPE items are
currently estimated to be being consumed each
day, amounting to a daily 15,000-ton mountain of
waste — much of which must be incinerated.
“We’ve done a lot of work with sustainable
polyamides and polyesters, as well as with PHAs
and a range of of PLAs,” Slack says. “In the
longer term, there has to be a more sustainable
option than polypropylene in these products and
the opportunity to explore potential
alternatives — drawing on the know-how from the
extensive body of tests and trials we’ve carried
out in the past, as well as the machines run
commercially by our customers — is something I
believe makes us pretty unique in the services
we can offer nonwovens manufacturers.
Conventional meltblown and spunbonded systems
are usually designed for high capacity systems
and are not suitable for product development, he
adds.
“They consume high quantities of materials and
as a consequence are not suitable for
development work with high value materials or
for niche applications. They also rely on
specially formulated low viscosity polymers
which is a further limitation which does not
apply to us.
In processing finer filaments, FET has achieved
structures with average mean filament diameters
of 1.68 microns and 58% of between 0.5 to 1.5
microns, in web thicknesses of 37 microns with
bulk density of 98 mg/ml and porosity of around
92%.
FET’s system is designed for the processing of
pure polymer with no need for processing aids or
additives.
“A wide range of structural and mechanical
properties are obtainable, with numerous options
for post-processing of the web, such as by
calendaring, point bonding or lamination,”
Richard Slack concludes. “More effective and
sustainable PPE solutions could well be achieved
through further product development.”
NXTNano has Nanofiber Media Available for Face
Masks
A recent independent test by a mask supplier
showed high efficiency for media made by NXT
Nano. The company currently has some capacity
available for manufacturing N95 rated face mask
material. However they released this statement
“Please understand this situation is fluid, and
that as COVID-19 continues we expect this
capacity to fill”.
Materials are nanofiber coated PET in ranges
from 29 to 70 GSM depending on the needs of
individual manufacturing lines.
In 2019 NXTNano, commenced
installation of its third HYPR-Spun Nanofiber
production line, bringing its annual capacity to
above 60 million square meters of high
efficiency air filtration media. Like NTXNano's
existing production lines, the newest equipment
will facilitate high volume nanofiber
manufacturing up to a maximum roll width of 2.15
meters.
“As we continue to see very strong demand in
nanofiber air filtration medias as well as our
apparel, medical, and microfiltration business
segments, we felt it was critical to keep
capacity ahead of demand so our customers can
continue to count on the fast order turns they
have become accustomed to,” says director of
sales Andrew McDowell.
Tustar Teams with Neatrition to Introduce High
Efficiency Masks to the U.S. Market
With the help of Ann Arbor, Michigan-based TusStar,
Chinese nanotechnology company Neatrition is
introducing new KN95 safety masks to the U.S.
market.
These easy-to-clean, multiple-use masks will
shield users from respiratory droplets and other
particulate matter during the current COVID-19
crisis and beyond.
Neatrition collaborated with Tsinghua
University, a major research university
in Beijing, to develop and create these new nano
medical masks that offer several advantages over
traditional medical masks. Made in labs
in China and overseen by academic researchers to
ensure clean standards and a top-of-the-line
product, the Neatrition KN95/NMV95 masks have
a unique micro-nano sharkskin structure and
bacteriostatic features that not only block
aerial droplets from getting in, but also
quickly kills viruses attached to the surface of
the mask. U.S. Lab testing is currently underway
for the anti-virus feature in which a virus will
become inactive, degenerate and die within 1
minute on the surface of the material.
These advanced protection devices feature a
strong droplet surface coating that allows the
masks to last up to 10 times longer. The masks'
soft sewn stretch knit ties and carefully
crafted inner mask design provides a comfortable
fit that is easy to wear for long stretches and
is exceptionally breathable.
Traditional protective masks use material that
can only be used once and only isolate droplets
from the air," said TusStar President Frank Ni.
"Unfortunately, many medical professionals and
mask wearers are then still infected by the
virus even after using these traditional masks
because the virus is still alive on the outside
surface."
A 5-mask package sells for $32, plus tax and
shipping. Quantities of 500 masks are available
at a discounted rate of $2,800.
Beijing Neatrition Technology Co., Ltd. was born
in Tsinghua University. It is a new material and
technology company integrating R & D, production
and sales (Neatrition has developed
superhydrophobic technology. The series products
mainly cover (super) hydrophobic nanomaterials,
which are waterproof, dustproof, snowproof,
oilproof, etc. Neatrition, as a frontier science
and technology enterprise in Zhongguancun, is
committed to solving the surface cleaning and
maintenance of all related objects for
industrial and other users, the efficiency of
its masks are compare to traditional N95-N100
masks.
|
Neatrition® nano medical mask |
Traditional medical mask |
Filtration Efficiency |
Virus survival time on the surface |
||
|
Test with NaCl particles |
Test with oil particles |
Neatrition® |
Traditional medical mask |
||
|
Neatrition® NM95(can be reused up to 10 times) |
KN95(can be reused up to 3 times) |
>90-95% |
-- |
<2min |
>2d |
|
Neatrition® NMV95(can be reused up to 20 times) |
N95(can be reused up to 3 times) |
>95% |
-- |
<2min |
>2d |
|
Neatrition® NM100(can be reused up to 20 times) |
N100(can be reused up to 3 times) |
>99.9% |
-- |
<2min |
>2d |
|
Neatrition® NTV100(can be reused up to 20 times) |
None |
>99.9% |
>99.9% |
<2min |
>2d |
Due to the anti-bacterial qualities the masks
can be stored and reused. One regimen is to
rotate masks every three days to insure
inactivation of the virus which was retained on
the mask surface.
Sciessent
Antimicrobial Used in Hanesbrands Masks
Sciessent has partnered with both healthcare and
non-healthcare manufacturers to develop and gain
regulatory clearance for masks containing Agion
Antimicrobial for use in healthcare settings.
Sciessent has helped two suppliers obtain
approval for the anti-microbial masks—one with
a traditional medical device manufacturer
following the severe acute respiratory syndrome
(SARS) outbreak in the early 2000s, and another
this year with a manufacturer from outside of
the medtech industry—comparing and contrasting
the two from an FDA regulatory process
perspective.
Following the 2003 SARS outbreak, medical device
manufacturer
Nexera made the decision to develop a N95
respirator mask constructed from Foss
Performance Materials’ Agion Antimicrobial
treated polyester fiber. As a medtech company,
Nexera had experience with FDA review for its
devices so it understood what the process
entailed. The company looked to Sciessent as its
supply partner to help with regulatory
considerations related to the antimicrobial.
Because Nexera had developed the mask in
response to SARS, as a product that could help
in future respiratory virus outbreaks, the
Nexera and Sciessent teams had ample time for
pre-planning prior to FDA submission. This
included the opportunity to develop data to
support the mask’s antimicrobial claims. Working
together, the teams successfully obtained FDA
clearance for Nexera’s SpectraShield 9500 N95
respirator mask, and later leveraged additional
data to secure an updated 510(k) with cleared
claims to inactivate viruses by 99.99 percent in
five minutes.
Sciesent also worked with Hanesbrands. While the
FDA is leveraging its Emergency Use
Authorization (EUA) to accelerate the timeframe
for clearance of products to address the
COVID-19 crisis, and this is certainly a benefit
to companies producing PPE, Hanesbrands still
had to meet the agency’s requirements. Sciessent
served as a collaborative partner in these
efforts with medical device expertise, a wealth
of data, and extensive experience in navigating
the FDA’s regulatory review pathway.
Unlike the premeditated work with Nexera to
develop a mask following the SARS outbreak
nearly two decades ago, work with Hanesbrands
required that the team leverage existing
knowledge and experience to quickly fill-in
various information gaps. Instead of developing
a product from the ground up, Hanesbrands was
repurposing its knitted cotton fabrics treated
with Agion Antimicrobial to develop its masks.
Sciessent supported Hanesbrands in their
interactions with FDA. Given the urgent need to
address the PPE shortage, it appeared
communication was far more open and frequent
with the agency under the EUA directive. The
correspondence and exchange of information
between Hanesbrands and FDA moved quickly. In
this circumstance, the FDA did not clear
specific efficacy claims; in fact, the agency
said no efficacy claims could be made.
Having worked closely with the FDA with Nexera
for its N95 mask and with other medical device
manufacturers for their products, Sciesent was
able to pivot quickly and apply its
understanding of the regulatory process to the
Hanesbrands situation. It was also able to
leverage the data on safety and efficacy,
including viruses from the Nexera mask’s second
510(k) clearance, to help fast track the
Hanesbrands mask clearance. As a result, FDA
cleared Hanesbrands’ mask in a matter of days,
enabling the company to quickly transition its
operations to relieving the PPE shortage.
Teho Filter Using Ahlstrom Media for N88 Masks
in Finland
Ahlstrom-Munksjö is supplying facemask material
from its plant in Tampere to Teho Filter for the
assembly of masks. The masks will be available
in May-June in the stores of Finland based
retailer S Group.
The filtration efficiency of the face mask
material of 88% has been verified by VTT
Technical Research Centre of Finland. It is
substantially higher than the roughly 20-40%
efficiency of masks made from cloth; the company
claims. The filtration efficiency of a
mechanical filter media remains intact over time
compared to electrostatically charged materials
the efficiency of which may decrease in humid
conditions, Ahlstrom-Munksjö adds.
“We work with determination in order to enhance
the supply of face masks for consumers in
Finland. At the same time, we are further
supporting the reopening in Finland once the
corona pandemic restrictions loosen,” states
Otto Kivi, Sales Manager at Ahlstrom-Munksjö’s
Filtration business.
The company announced in April that it would
start the production of facemask materials in
Tampere, where its plant is capable of producing
material for more than 10 million masks in the
short-term and has the ability to increase
capacity to about 30 million masks per month.
Earlier in May, the company announced a similar
co-operation on facemask supply with other
Finnish players.
” We are also very pleased about the continued
product development in Tampere: our production
now meets the filtration efficiency requirements
of surgical face mask materials. Currently, we
are working together with our customers to
develop a product that meets the European
Standard EN 14683 for surgical masks. The
availability of various protective gear is
vitally important,” Kivi continues.
Ahlstrom-Munksjö has long-term and in-depth
knowledge in the production of protective
materials for the healthcare industry. Due to
the coronavirus pandemic, the company has
increased its offering in protective fabrics and
expanded the production of face mask materials
globally to support the healthcare sector.
Russian Scientists Develop Nanofibers for Masks
A group of scientists from Russia’s Krasnodar
Region invented
a nanofiber material for production of medical
masks and respirator disposable filters that is
more effective for prevention of the coronavirus
spread than normal cloth,
Dmitry Lopatin, a team representative, told TASS
recently.
"We have begun our research in late March:
kerosene soot particles have the same size as
the coronavirus. We tested both the normal cloth
used in mask production and our nanofibers for
absorption. Currently, we are looking into two
possible applications of our nanofibers for
coronavirus prevention: production of medical
masks and disposable respirator filters,"
Lopatin said. Dmitry Lopatin is a graduate of
the Kuban State University. The group conducts
its research in the Krasnodar Region’s Goryachy
Klyuch.
O2 Nano Mask is Low Pressure Drop, Efficient and
Reusable
Viaex Technologies, a material science startup
and laboratory, has been researching
nanomaterials and developing their own products
for over six years. They have collaborated with
DaD Sewing House, a network of local
manufacturers that provide employment
opportunities to skilled craftspeople in San
Francisco, to create the O2 Nano Mask.
Distributed under O2
Brands,
the O2 Nano Mask was created in April 2019 as a
high-quality pollution mask, and this year, its
production was increased to meet the demand of
communities and healthcare facilities for protective
masks for
health workers and people affected by Covid-19.
O2
The O2
Nano Mask is sold at $35.99 and
comes in medium and large sizes.
The mask consists of two components: the
reusable skin and the replaceable filter.
Each filter features three layers: two layers of
PET and one embedded with nanofiber material.
The fibers are on the order of 85 ± 20 nm in
diameter. This nanoscopic morphology creates
uniquely small pores and drastically increases
the membrane surface area while leaving open-air
travel paths in 99% of the membrane volume.
The O2 Nano Mask’s filter component stops 99% of
PM2.5 particles, including dust and other air
pollutants; pollen; dander and other allergens;
bacteria and other germs; harmful gases; smoke
(including from wildfires) and other fumes with
its five-layer protection and hypoallergenic
hydrophobic nanofilter. The filter media used
has achieved 99% particle removal efficiency
based on EN143 European certification standards
and third-party testing.
The mask’s outer shell component can last for a
long time. The mask is machine washable and
tumble dryer safe. It’s intricately constructed
to be worn time and time again. The filters are
long-lasting depending on the level of pollution
and are also fully recyclable. Each O2 Nano Mask
filter can last from eight hours to 20 days
(with light exercise in a moderate AQI
environment).
Cambridge has an Efficient and Comfortable Mask
but with Valve
The pro mask uses a unique triple filter system
which has been tested by Nelson and reaches N99
efficiency levels.
The first layer of the filter system catches
larger pollution particles such as dust and
PM10. It’s then backed up by the Three-Ply Micro
Particulate which stops nearly 100% of smaller
particulate matter such as PM2.5 and PM3.0.
The
inner filtration layer is made from 100% pure
activated carbon cloth, originally developed by
the UK Ministry of Defense for chemical,
biological, and nuclear warfare protection.
The carbon filter is treated with silver to
ensure 99% of harmful pathogens are removed and
killed. The filter material is comprised of a
series of activated carbon filaments, each about
2,000 nanometers in diameter. Each filament is
many times smaller than the typical grain size
in standard carbon materials, making the rate of
adsorption of pollutant gas much faster and
therefore more powerful. It also means that
bacteria and viruses are drawn to the filament
surface much more efficiently, because there is
so much more available surface than in a
granular carbon.
The high number of filaments – spun into a yarn
and then woven into cloth – makes the speed of
adsorption extremely fast in a material that is
still easy to breathe through. Not only are
molecules such as pollutant gases and endotoxins
quickly adsorbed into the pores from a much
wider area, but the Van der Waals forces also
attract and immobilize on the filament surface
much larger particles including bacteria, which
often have a negatively charged membrane.
Together with the anti-bacterial silver added to
the filament surface, the activated carbon cloth
traps the bacteria and draws out the gel-like
cytoplasm inside – killing it and preventing
infection.
Cambridge uses
British military technology developed for
chemical, nuclear, and biological warfare in the
mask that’s manufactured in the UK. But the
masks are assembled in Indonesia and China.

Masks Performance Comparison
|
Fashion friendly |
✔ |
✘ |
✘ |
|
Suitable for
children |
✔ |
✘ |
✘ |
|
More
environmentally
friendly
long-lasting
mask |
✔ |
✘ |
✘ |
|
Comes with high
quality
cardboard for
safe and easy
carrying |
✔ |
✘ |
✘ |
|
Adjustable
earloops |
✔ |
✘ |
✘ |
|
Blocks over 99%
of viruses and
bacteria |
✔ |
✘ |
✘ |
|
Filters gas
pollutants such
as Ozone and
oxides |
✔ |
✘ |
✘ |
|
Mask filter life |
200-300 hours
(3-6 months) |
8-10 hours |
8-10 hours |
|
Cost per hour of
use (USD) |
$0.08c |
$0.23c |
$0.03c |
Draeger has Long Term Contract to Deliver N95
Masks to HHS
The U.S. Department of Health and Human Services
(HHS) issued an award to Dräger for the supply
of National Institute of Occupational Safety and
Health (NIOSH) approved N95 respiratory
protection masks at the end of March. The
contract is part of the recent government
announcements concerning the supply of masks. As
part of the contract Dräger plans to increase
U.S. domestic production of the masks and expand
its manufacturing footprint over the course of
the contract. Deliveries will take place over
the next 18 months with a focus on accelerated
supply wherever possible.
“Respiratory protection has been at the core of
our capabilities for over 110 years. Our N95
mask design offers superior comfort and
breathability and is certified to the NIOSH
standard for particle respiratory protection,”
said President and CEO for Dräger in North
America, Lothar Thielen. “This contract comes in
addition to the ongoing work we are doing to
protect frontline personnel in the fight against
the pandemic. We are humbled to be able to
support healthcare professionals and first
responders with our Technology for Life, which
spans both healthcare and safety applications.
In addition we are proud to further increase our
U.S. investments and manufacturing base in
support of this contract.”
Dräger is an international leader in the fields
of medical and safety technology. Our products
protect, support, and save lives. Founded in
1889, Dräger generated revenues of almost EUR
2.8 billion in 2019. The Dräger Group is
currently present in over 190 countries and has
more than 14,500 employees worldwide
CDC Approves Powered Air Purifying Respirators
NIOSH-approved respirators are available in many
types, models, and sizes from many manufacturers
for a wide variety of uses in many occupational
settings. The most common types of respirators
in healthcare are N95 filtering facepiece
respirators (FFRs), surgical N95 FFRs, and
PAPRs.
Of these three options, many healthcare
practitioners are the least familiar with PAPRs.
A PAPR is an air-purifying respirator that uses
a blower to force air through filter cartridges
or canisters and into the breathing zone of the
wearer. This process creates an air flow inside
either a tight-fitting facepiece or
loose-fitting hood or helmet, providing a higher
assigned protection factor (APF) than the
reusable elastomeric non-powered air-purifying
half facepiece (half mask) or N95 FFRs. A PAPR
can be used for protection during healthcare
procedures in which HCP are exposed to greater
risks of aerosolized pathogens causing acute
respiratory infections.
A PAPR may have a tight-fitting half or full
facepiece or a loose-fitting facepiece, hood, or
helmet. It has an OSHA APF of at least 25 for
loose-fitting hoods and helmets, 50 for
tight-fitting half masks, and 1,000 for full
facepiece types and some loose-fitting hoods and
helmets where the manufacturer’s testing has
demonstrated an APF of 1,000.
CDC has published recommendations for HCP
respiratory protection and of commonly used
NIOSH-approved, FDA-cleared, single-use
filtering facepiece N95 surgical respirators.
Properly fitted FFR and half facepiece reusable
elastomeric respirators are expected to reduce
exposures to one-tenth of the concentration that
is in the air, based on OSHA’s APF of 10 for
these respirator types. All PAPR APFs exceed the
APF of 10 for N95 FFR or elastomeric half
facepiece respirators.
PAPRs reduce the aerosol concentration inhaled
by the wearer to at least 1/25th of that in the
air, compared to a 1/10th reduction for FFRs and
elastomeric half facepiece air-purifying
respirators. OSHA assigns an APF of 1,000 to
some PAPRs with hoods or helmets. However,
employers must have evidence provided by the
respirator manufacturer that testing of these
respirators demonstrates performance at a level
of protection of 1,000 to receive an APF of
1,000. Absent such evidence, PAPRs with
loose-fitting helmets or hoods have an APF of
25. When used properly, PAPRs provide increased
protection and decrease the likelihood of
infection transmission to the wearer as compared
to FFRs and half face reusable elastomeric
respirators.
A variety of NIOSH-approved PAPR designs are
available. Examples include those with
tight-fitting facepieces and loose-fitting hoods
or helmets, blower styles, battery types (e.g.,
Lithium ion, Nickel-Metal hydride, Nickel
Cadmium) or over-the-counter disposable
batteries, and high efficiency (HE) filters or
filter cartridges. HE filters are 99.97%
efficient against 0.3 micron particles. A PAPR
may have adjustable air flow rates for added
comfort and a range of cartridge protections
some of which are solely for particulates (HE
filters) and others which also protect against
chemical gases and vapors that can be used to
help protect against hazards associated with the
handling of certain hazardous drugs and
cleaning/disinfecting operations. The
substantial PAPR product diversity provides
flexibility to customize protection needed in a
healthcare setting.
IQ Mask Uses HEPA Grade Filtration Media
IQAir Mask features a unique
exhaust valve design with an ultra-thin
valve membrane for low air resistance and
immediate air exchange. Many air pollution masks
use ineffective material that wears out quickly
due to moisture build-up and high filter
material resistance – IQAir Mask ensures an even
exchange of inhaled clean air and exhaled
breaths while reducing moisture and CO2
build-up inside the mask to prevent drowsiness,
headaches, or loss of energy.
|
|
IQAir Mask |
|
|
|
|
General Features |
|
|
Air leak
protection |
SoftSeal
prevents air
leakage with
minimal pressure
on facial skin |
|
Filter media |
Melt-blow cage
(PE) |
|
Breathing valve |
FeatherValve for
CO2
release upon
exhalation |
|
Mask structure |
3D Filter Dome
for space
between media
and skin |
|
Sealant material |
Terylene
polyester fiber |
|
Product lifespan |
3-year
shelf-life
(recommended) |
|
Suggested time
for replacement |
When the mask is
broken, stained,
or the
respiratory
resistance |
|
Color |
White filter
material, gray
sealant fabric
and logo imprint |
|
Storage
temperature |
< 70% rel. H,
-4°F to 158°F
(-20°C to +70°C) |
|
Operating
temperature |
4° to 140° F
(-20 to +60 °C) |
A Mask is Not a Mask-Big Difference in
Efficiency and Fit
An analysis of mask filter media shows that
there are big differences between materials and
the fit of the mask.
Homemade masks can be quite efficient on
removing particles such as viruses but the
pressure drop is high. The fit of the mask means
as much as the media efficiency.
Details of the study are shown in an ACS
publication
https://pubs.acs.org/doi/10.1021/acsnano.0c03252
There is a need to evaluate filtration
efficiencies as a function of aerosol
particulate sizes in the 10 nm to 10 μm range,
which is particularly relevant for respiratory
virus transmission. The researchers carried out
these studies for several common fabrics
including cotton, silk, chiffon, flannel,
various synthetics, and their combinations.
Although the filtration efficiencies for various
fabrics when a single layer was used ranged from
5 to 80% and 5 to 95% for particle sizes of <300
nm and >300 nm, respectively, the efficiencies
improved when multiple layers were used and when
using a specific combination of different
fabrics. Filtration efficiencies of the hybrids
(such as cotton–silk, cotton–chiffon,
cotton–flannel) was >80% (for particles <300 nm)
and >90% (for particles >300 nm). The
researchers
speculate that the enhanced performance
of the hybrids is likely due to the combined
effect of mechanical and electrostatic-based
filtration. Cotton, the most widely used
material for cloth masks performs better at
higher weave densities (i.e.,
thread count) and can make a significant
difference in filtration efficiencies. Studies
also imply that gaps (as caused by an improper
fit of the mask) can result in over a 60%
decrease in the filtration efficiency, implying
the need for future cloth mask design studies to
take into account issues of “fit” and leakage,
while allowing the exhaled air to vent
efficiently. Overall,
the study
finds that combinations of various
commonly available fabrics used in cloth masks
can potentially provide significant protection
against the transmission of aerosol particles.
Table 1. Filtration Efficiencies of Various Test
Specimens at a Flow Rate of 1.2 CFM and the
Corresponding Differential Pressure (ΔP)
across the Specimens
|
flow rate: 1.2 CFM |
|||
|
filter efficiency (%) |
pressure differential |
||
|
sample/fabric |
<300 nm average ± error |
>300 nm average ± error |
ΔP (Pa) |
|
N95 (no gap) |
85 ± 15 |
99.9 ± 0.1 |
2.2 |
|
N95 (with gap) |
34 ± 15 |
12 ± 3 |
2.2 |
|
surgical mask (no gap) |
76 ± 22 |
99.6 ± 0.1 |
2.5 |
|
surgical mask (with gap) |
50 ± 7 |
44 ± 3 |
2.5 |
|
cotton quilt |
96 ± 2 |
96.1 ± 0.3 |
2.7 |
|
quilter’s cotton (80 TPI), 1 layer |
9 ± 13 |
14 ± 1 |
2.2 |
|
quilter’s cotton (80 TPI), 2 layers |
38 ± 11 |
49 ± 3 |
2.5 |
|
flannel |
57 ± 8 |
44 ± 2 |
2.2 |
|
cotton (600 TPI), 1 layer |
79 ± 23 |
98.4 ± 0.2 |
2.5 |
|
cotton (600 TPI), 2 layers |
82 ± 19 |
99.5 ± 0.1 |
2.5 |
|
chiffon, 1 layer |
67 ± 16 |
73 ± 2 |
2.7 |
|
chiffon, 2 layers |
83 ± 9 |
90 ± 1 |
3.0 |
|
natural silk, 1 layer |
54 ± 8 |
56 ± 2 |
2.5 |
|
natural silk, 2 layers |
65 ± 10 |
65 ± 2 |
2.7 |
|
natural silk, 4 layers |
86 ± 5 |
88 ± 1 |
2.7 |
|
hybrid 1: cotton/chiffon |
97 ± 2 |
99.2 ± 0.2 |
3.0 |
|
hybrid 2: cotton/silk (no gap) |
94 ± 2 |
98.5 ± 0.2 |
3.0 |
|
hybrid 2: cotton/silk (gap) |
37 ± 7 |
32 ± 3 |
3.0 |
|
|
|
|
3.0 |
The quilter cotton mask could have a capture
efficiency of less than 13%. This means that
these masks are not effective in combatting the
virus. Some of the fabrics which are effective
have a high pressure drop. Most important the
fit of the mask is a key factor. An N95 mask
with a gap is only 34% efficient on particles
less than 0.3 microns.
The advice about wearing masks tends to treat
all masks as equal.
In fact there is a world of difference.
W.L. Gore has a Number of Products it is
Developing to Fight COVID
Gore has engineered prototype reusable mask
covers to supplement clinicians’ primary face
masks.
These covers, developed by a cross-divisional
team, are made from a material that:
·
is a proprietary Gore high-flow filtration
laminate
·
provides greater than 99% aerosolized virus
particle protection
·
is water repellent yet air permeable, and
·
can be reused after autoclave or EtO
sterilization
This effort went from a product concept to
prototypes in less than one week. Gore currently
has prototypes being evaluated at a limited
number of U.S. facilities in COVID-19 outbreak
hot spots. Based on feedback from those piloting
the prototype, Gore intends to optimize the PPE
Protector design and then scale up production
for broader distribution.
Examples of other initiatives underway include:
·
Protective medical gowns, using fabric laminates
from current inventory
·
Universal filter cartridge prototypes for use in
respirators, hoods and ventilators that
incorporate Gore filtration materials intended
to provide N95 particulate protection
·
Disposable N95 respirators, using Gore
filtration laminates
N80 Masks Should be Worn by Three Billion People
Who are in Public Space Every Day.
In a conversation with Dave Rousse of INDA
earlier this week he talked about the N80
concept.
This gives a specific name to what he
believes will be a critical mission to assure
that everyone in a public space is wearing an
efficient mask. In our previous Alerts we have
written about the
Berry Development of a mask in this
quality range.
We further covered it in a Berry Profile.
LINK to Berry Power Points
We also reported earlier about Ahlstrom-Munksjö,
whose products Reliance SMS 200, Reliance SMS
300, Reliance Dextex 200 and Reliance
Dextex 300 have been declared compatible with
the French requirements for face masks used by
civil servants in contact with the public. The
material is typically used for the manufacture
of sterilization wraps for surgical instruments.
Reliance SMS 200 and Reliance SMS 300 have also
been tested compatible with the European
Standard EN 14683, meeting the performance
criteria of surgical masks.
This month the
company is producing
material equivalent to 100 million face
masks for civil servants in contact with the
public such as police officers, prison
administrators and social workers as well as
companies active in essential sectors such as
food, energy, water and waste.
“The attainment
of this long-term work for the selection of our
products represents a moment of collective pride
at Ahlstrom-Munksjö. Our medical business serves
the medical device market worldwide all year
round, and our agility and ability to innovate
makes us an ideal partner in critical
situations,” says Lionel Bonte, vice president
of medical business.
Modern Healthcare Corporation has N-80 Mask
Motex, manufactures disposable medical surgical
wound dressings and medical tapes as well as the
varied face masks in Taiwan. It has
plants located in Thailand, Shanghai and
Taiwan. They
have the certificates of ISO, CE, GMP.
and some items with US FDA510K approved. The
company was established in 1978 and has less
than 100 employees.
It offers N80 masks. This designation is being
used by those who are offering a mask for the
general population which is higher efficiency
than most masks being worn by the public but
would be 80% efficient on 0.3 micron particles
in contrast to the 95%
N95.

The original name of the company
was Huaxin Medical Materials. It
has won awards for a
new airtight protective
mask. The overall safety and
comfort of flat masks have
greatly improved. Chairman Zheng
said that the patented
technology has the advantages of
integrated design, fully
automated production, cost
competitiveness, etc., and can
be applied to various flat masks
on the market such as: dustproof
(protective) masks, activated
carbon masks, surgical bandage
masks ... I believe that we can
recreate the blue ocean business
opportunities in the mask market
and benefit the consumers.
Masks are Part of a Combined
Program to Reduce the Odds to
Near Zero
Ryanair CEO Michael O'Leary has claimed that if
everyone wore face masks on planes and public
transport, it would ''eliminate the risk of
spreading Covid-19 by about 98.5%''.
O'Leary, who wants to restart flights in July,
was speaking on BBC Radio 4's Today programme.
He called the government's plan to quarantine
travelers for 14 days "ineffectual" and
"unmanageable".
O'Leary said the 98.5% figure came from a study
by the Mater Hospital in Dublin.
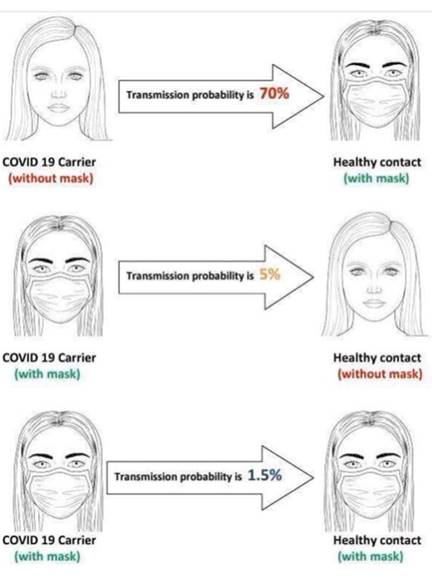
This is over simplified because
the efficiency of each mask is
not specified. But if both are
wearing N95 masks the
probability should be reduced to
fractions of a percent.
This could be reduced to close
to 0 with partitioning.

There is already an air nozzle
above each passenger.
If this is directed
downward you have the ideal
cleanroom conditions of downward
laminar flow of HEPA filtered
air.
The avoidance of COVID-19 is a gamble but one where each safety measure keeps increasing the odds. If there is screening of passengers and temperature checks at the gate. The odds of sitting next to a COVID carrier are small. This would be increased further if passengers were tested for COVID before they boarded.
Airplanes are Relatively Safe with the Following
Guidelines
Joseph Allen, assistant professor of exposure
assessment science at Harvard, argues that
despite what you may think, “you don’t get sick
on airplanes more than anywhere else.”
Allen says airlines have, for many years, worked
to keep passengers safe from disease while they
travel.
“The ventilation system requirements for
airplanes meet the levels recommended by the
Centers for Disease Control and Prevention for
use with COVID-19 patients in airborne infection
isolation rooms,” Allen said
Midwest Textiles, Hollingsworth & Vose Partner
to Develop Homemade Facemask Kit
Midwest Textiles and Hollingsworth & Vose (H&V)
are collaborating on a new ready-to-sew face
mask kit for the general public. The new
collaboration between Midwest and H&V offers an
improvement to the everyday consumer by adding a
layer of Nanoweb FM to the mask.
Nanoweb FM is new filtration media made by H&V,
designed for use in homemade face masks.
“H&V is one of the world’s leading producers of
filtration media for face masks and many other
filtration applications. By partnering with
Midwest, and through the development of Nanoweb®
FM media, we are able to help support
individuals and communities across the country
that are struggling to obtain basic levels of
protection,” said Mike Clark, Division President
at H&V. “Our new Nanoweb® FM media was designed
specifically for general use in homemade face
masks and can be inserted in a face mask pocket
or stitched into a disposable pleated mask.”
Consumers can purchase ready-to-sew face mask
kits and Nanoweb® FM media for homemade masks
at www.sitnsewfabrics.com.
One kit containing 4 masks will cost $24.95, and
it is estimated to take 15 minutes to sew and
assemble each mask.
Bondex Ramps up Production of Material for PPE &
N95 Facemasks
To support the industry demand for PPE
materials, Bondex, a producer of carded thermal
bond, hydroentangled and needlepunch nonwovens,
is dedicating a portion of its capacity to
produce materials designed for use in N95 mask
construction and materials for use in isolation
gowns and other PPE applications.
Bondex develops a polypropylene nonwoven that is
used in both mask and isolation gown
applications, as well as hydroentangled
polyester that is also used in the construction
of mask materials.
“We have implemented plans as necessary to
continue our supply to our customers though the
pandemic crisis,” says Bondex president Brian
Little. “We have also recognized the needs in
society to help battle this COVID-19 pandemic so
we are adding staff in order to supply technical
nonwovens for selected PPE applications in order
to support these key initiatives.”
Ultra-Pure PM 0.1 Mask Filter from Asiatic Fiber
Corporation
The mask filter has three layers, and each layer
has its unique purpose. The outer layer is
air-droplets blocker, that can preliminarily
filter the majority of particles and
air-droplets. The
second layer is an AFC® filter pad, it embraces
air-in area and air-out valve, to create an PM
2.5 filtration effect. The base layer is
anti-bacterial layer, and it also brings
anti-odor and anti-static effect (to diminish
the adherence of particle). The anti-bacterial
is a long-lasting and durable for several times
of laundry. This mask filter is being tested
that can effectively block, filter and form an
excellent barrier of air-droplets,
micro-particle, bacterial, dust, pollen and smog

Features:
-
Triple filtration, BFE &
PFE ≥ 99%, tested by Nelson labs
-
PM 2.5 level, effectively
filtering micro-particle and
smog
-
Centralized air exchange area,
to enhance filtering effect
-
Exceptional air valve design,
brings comfort without
sweltering
-
Long-lasting anti-bacterial,
remains 99% even after 50 times
laundry
-
Multi-functional fiber, brings
anti-bacterial, anti-odor and
anti-static (less particle
adherence)*BFE = Bacterial
Filtration Efficiency; PFE =
Particle Filtration Efficiency
*PM = Particulate Matter
Ahlstrom -Munksio Media Being Used by French
Mask Makers
Ahlstrom-Munksjö products Reliance SMS
200, Reliance SMS 300, Reliance Dextex 200
and Reliance Dextex 300 have been declared
compatible with the French requirements for face
masks used by civil servants in contact with the
public. The material is typically used for the
manufacturing of sterilization wraps for
surgical instruments. Reliance SMS 200 and
Reliance SMS 300 have also been tested
compatible with the European standard EN 14683,
meeting the performance criteria of surgical
masks.
During May the
company will produce material equivalent to 100
million face masks for civil servants in contact
with the public such as police officers, prison
administrators and social workers as well as
companies active in essential sectors such as
food, energy, water and waste.
“The attainment
of this long-term work for the selection of our
products represents a moment of collective pride
at Ahlstrom-Munksjö. Our medical business serves
the medical device market worldwide all year
round, and our agility and ability to innovate
makes us an ideal partner in critical
situations,” says Lionel Bonte, vice president
of medical business.
In France,
Ahlstrom-Munksjö has some 1600 employees,
eight production sites, two global R&D centers
and a sales office. Measured by the amount of
employees, France is the second largest country
of operations representing 20% of gross sales.
Ava Breathe has Unique Mask Design
One Start X Med company, AVA Breathe, has taken
a look at the various masks and face coverings
out there and determined there’s plenty of room
for improvement.
“So most people have paper masks or cloth masks
and things that are poorly fit and don’t
actually maybe properly protect people from this
current COVID crisis,” said Eric Sokol,
Co-Founder of AVA Breathe. “So
we developed a small personal air purifier that
you can wear underneath it. So this is really
the world’s smallest N90 filter. When coupled
with a surgical or cloth mask, it provides a lot
of protection, along with sophisticated health
monitoring.” That includes the ability to
monitor a user’s respiratory rate, respiratory
pressure and body temperature.
Sokol is a Stanford professor and physician, who
teamed up with two other Stanford professors, to
found AVA Breathe and enter StartX. The product
also addresses the leak problem.
“We can make this as a little stick on filter,
clip on filter or it could be embedded into any
high-end mask,” said Sokol. “So you can adjust
then your mask so that it’s properly fit and
working for you to protect you.”
Sokol says while their products provide
immediate protection and detection, it may
ultimately be the data collected that someday
helps predict how your body reacts to the air
you breathe. The startup AVA Breathe spun out of
a Stanford Biodesign program and later joined up
with StartX.
High Fashion Mask is Available from Lumen
Couture
A
$95 LED Matrix Face Mask
allows wearers to write their own text:
draw designs or use a
phone’s microphone or music tracks for
equalizer effects. The construction is a
Dual-layer cotton and mesh material with LED
Flex Panel. It is washable. Electronics are
removable for cleaning and sanitation. This is a
novelty/fashion mask and not tested for medical
efficiency nor does it make claims for medical
protection. The tech components can be removed
for normal wear and better air circulation.
Battery and charge cord included.

This mask is not efficient enough to provide
maximum protection.
However, it does show the potential for
fashionable masks.
Website Dedicated to Analyzing Mask
Decontamination Options
A team of 60 scientists and engineers, students
and clinicians, drawn from universities and the
private sector, are unveiling N95decon.org,
a website that synthesizes the scientific
literature about mask decontamination to create
a set of best practices to decontaminate and
reuse this protective face covering during the
current emergency.
“While there is no perfect method for
decontamination of N95 masks, it is crucial that
decision-makers and users have as much
information as possible about the strengths and
weaknesses of various approaches,” said Manu
Prakash, an associate professor of
bioengineering at Stanford
who helped coordinate
this ad hoc, volunteer undertaking. “We aim to
provide information and evidence in this
critical time to help those on the front lines
of this crisis make risk management decisions
given the specific conditions and limitations
they face.”
The team members who came together over the last
few weeks scoured hundreds of peer-reviewed
publications and held continuous online meetings
to review studies of decontamination methods
that have been used on previous viral and
bacterial pathogens and then to assess the
potential to use these methods on the novel
SARS-CoV-2 virus that causes COVID-19.
Their goal was to provide overwhelmed health
officials with reliable, pre-digested scientific
information about the pros and cons of three
decontamination methods should local shortages
force a choice between decontamination and reuse
or going unmasked.
The three methods involve either heat and
humidity; a specific wavelength of light called
ultraviolet C (UVC); or treatment with hydrogen
peroxide vapors (HPV).
The scientists did not endorse any one method
but instead sought to describe the circumstances
under which each might be effective against the
virus provided rigorous procedures were
followed. They concluded, for instance, that
devices that rely on heat are effective under
specific temperature, humidity and time
parameters. With UVC devices, the group advised
making sure masks are properly oriented to the
light so the entire surface is bathed in
sufficient energy. They also found that the HPV
method could potentially be used to
decontaminate masks in volume – a recommendation
that is backed by the U.S. Food and Drug
Administration, which has already certified
certain vendors to offer hydrogen peroxide vapor
treatments on a large scale.
N95decon.org will
help facilitate the rapid deployment of these
emergency measures by pointing decision makers
to sources of reliable and detailed how-to
information provided by other organizations,
institutions and commercial services. For
example, the U.S. Centers for Disease Control on
Tuesday released a data-driven
fact sheet and a detailed overview for
implementing the same three decontamination
methods.
Prakash and his collaborators stressed that
decontamination does not solve the N95 shortage
and expressed hope that new masks will be made
available to health care workers and first
responders in large numbers as soon as possible.
N95 Masks Much Safer than Medical Masks for
Wearers
One of the conclusions of the N95decon group
relative to mask performance is displayed on the
website. Medical and cloth masks not only reduce
respiratory droplet transmission to the outside
from the wearer
but also reduce the number of particles
that reach the inside of the mask from the
outside, thereby protecting the wearer (van der
Sande et al., 2008). One carefully controlled
study (Lai et al., 2012) was done with
mannequins, where one expelled respiratory-like
particles at 1 or 2 feet from another mannequin
that wore either a fully-sealed or a mask with
gaps under the eyes or on the sides (e.g. a
medical-type mask). The number of particles
‘inhaled’ by the masked mannequin was measured
to assess protection. The fully-sealed mask
provided >97% protection under all conditions,
including a mimicked cough at a short distance.
The medical mask provided between 30% and 100%
protection, depending on the number of gaps
between the mask and the face, for how long the
other mannequin was expelling particles, and the
distance from the other mannequin. Another study
with mannequins used influenza viral particles
and found that medical masks blocked > 50% of
infectious viral particles that were breathed
in.
Avantor in Unique Position To Supply Products to
Mitigate the Coronavirus
In 2018 Avantor acquired VWR for approximately
$6.4 billion cash, in a deal the companies said
would create a combined global provider
of consumables-focused solutions and services to
life sciences and advanced technologies
businesses, as well as education, government,
and research institutions.
The deal was designed to enable the combined
company to serve customers from research through
production by joining Avantor’s strengths in
cGMP manufacturing processes and significant
exposure to emerging markets with VWR’s focus on
providing product and service solutions to
laboratory and production customers in the
Americas and Europe.
VWR supplies masks, gowns, gloves and other
consumables.
It also supplies hardware from various
manufacturers such as clean benches from
Bassaire (covered in the previous article).
VWR® Advanced Protection Face Masks offer
excellent bacteria and particulate protection
and are available in multiple styles to provide
security and comfort throughout extended use.
-
Guards against bacteria and
particulates
-
Highly breathable and fluid
resistant
-
Malleable noseband ensures
custom, secure seal
-
Soft and hypoallergenic
Masks feature three-ply construction for
excellent particle and bacterial filtration
efficiency.
Available in two styles, with spandex ear loops
or polypropylene ties ultrasonically welded to
maintain softness and protect against
particulates. An encased, 12cm (43/4")
malleable steel nose-band creates a secure
facial seal and improves wearer comfort.
Latex-free. Masks are rigorously tested and
manufactured in an ISO Certified facility under
stringent process controls to ensure that each
product meets exacting quality standards.
Products are validated through independent lab
testing.
|
Description |
Color |
VWR Catalog Number |
Unit |
Price |
|
Mask with Ear Loops |
Green |
414004-663 |
Case of 500 |
$236.78 |
|
Pallet of 30000 |
$14,164.42 |
|||
|
Mask with Ear Loops |
Blue |
414004-662 |
Case of 500 |
$236.78 |
|
Pallet of 20000 |
$9,442.94 |
|||
|
Mask with Ear Loops |
Yellow |
414004-665 |
Case of 500 |
$231.95 |
|
Pallet of 25000 |
$11,593.12 |
|||
|
Mask with Ear Loops |
Pink |
414004-664 |
Case of 500 |
$232.13 |
|
Mask with Ties |
Blue |
414004-666 |
Case of 500 |
$235.62 |
|
Pallet of 18000 |
$8,472.51 |
Upscale KN95 Masks Available
We have previously written about Vogmask with
designer type high efficiency washable masks
selling for $33. Here is mask which sells for
around $5 from 22 Mask.
The mask complies
with national standard GB2626-2006.
Filtration efficiency is not less than 95%.
Effective Protection: 5 layers of protection,
disposable dust mask, activated carbon layer,
non-woven fabric layer, double electrostatic
absorption cotton and soft non-woven fabric
layer.
Can filter tiny dust, pollen, particulate matter
and almost 95% of particles in the air.

https://22mask.com/masks-sale/kn95-mask-protection-efficency-95-anti-particle-mask-10-pcs/
Do we Need Masks with Valves for Certain People
and Certain Situations?
Two Chinese boys dropped dead within a week of
one another while wearing face masks during gym
class. The students, who were both 14, were each
running laps for a physical examination test
when they suddenly collapsed on the track. One
student at Changsha’s Xiangjun Future
Experimental School in Hunan province had been
wearing an N95 respirator and running a
1,000-meter exam when the fatal incident
occurred. It’s unclear whether an autopsy had
been ordered.
Though it’s not known whether the masks played a
role in either death, several schools in Tianjin
and Shanghai have canceled physical education
exams, according to the report.
These incidents raise the question as to whether
N95 masks with valves should be substituted for
those without valves under certain conditions.
One would certainly be heavy exertion.
Another would be individuals who have underlying
conditions e.g. asthma where breathing is more
difficult that it would be for the average
person.
Chinese N95 Masks Shipped to Massachusetts did
not Meet Requirements
A number of N95 facemasks from Chinese suppliers
were tested by MIT as requested by the State of
Massachusetts. Efficiency varied from 95% down
to as low as 28%. Many of the suppliers
delivered masks which were less than the 95%
efficiency.
Details of individual tests are shown in
the link.
https://www.mass.gov/doc/kn95-respirator-test-results/download
Alpha Pro Tech Expands Production of Face Masks
Lloyd Hoffman, President and CEO of Alpha Pro
Tech, said that orders for the face mask in
recent months
have been roughly 24 times the revenue
that the company has recorded from sales of this
product for each year since 2016.
“We have already commenced additional production
of our N-95 face mask at our Salt Lake City,
Utah facility, and we expect to continue to
increase our production capacity,” added
Hoffman.
“Despite our rapid increase in production
capacity, the backlog of orders continues to
expand, in dollar amount and fulfilment time. We
expect to fulfil approximately 30% of the
currently booked orders in the first quarter of
2020, and we expect further revenue growth after
the first quarter as additional orders are
shipped,” Hoffman explained. “We are committed
to allocating the necessary resources and
procuring the necessary raw materials in an
effort to meet this unprecedented demand for our
N-95 face mask and to aid communities around the
world as they address this ongoing healthcare
crisis.”

The Alpha Pro Tech N-95 Particulate Respirator
face mask’s unique flat-fold design features a
Positive Facial Lock (PFL) and meets the Centers
for Disease Control and Prevention’s (CDC) and
National Institute for Occupational Safety and
Health’s (NIOSH) recommended protection levels
for many airborne contaminants.
The N-95 face mask filters at least 95% of
airborne particles. The integrated Magic Arch
technology creates a comfortable breathing
chamber within the N-95 face mask by holding it
away from the wearer’s nose and mouth.
Alpha Pro Tech, Ltd is the parent company of
Alpha Pro Tech, Inc and Alpha ProTech Engineered
Products, Inc. The company develops,
manufactures and markets innovative disposable
and limited-use protective apparel products for
the industrial, cleanroom, medical and dental
markets.
Masks with Valves have Advantages but Design and
Maintenance are a Challenge
Paul Gardner, former chief of the Army's
Edgewood Chemical Biological Center (ECBC)
Respiratory Protection Branch was asked by
McIlvaine to use his experience in evaluating
new filter media for the Army and comment on
recent coronavirus needs. His analysis was
included in the April 27 Alert.
He discussed challenges such as the
higher breathing resistance, moisture, and
comfort concerns.
So we asked Paul to comment on the use of
masks with valves which eliminate some of these
challenges. Here is his response.
Paul:
The obvious benefit of exhalation valves used in
some FFRs is that they reduce the exhalation
resistance and moisture within the mask thus
improving overall comfort. As you
mentioned, they are suitable for protecting the
wearer as opposed to those around you should you
be contagious.
One tradeoff is the added expense to
manufacture. The biggest downside, however, is
that they are potential leak sources and in a
reusable FFR would need to be checked and
maintained after each use to ensure they are
free of contaminants and functioning
properly. There is the added risk that people
will not perform the maintenance to ensure the
valve is clean, undistorted, and seated
properly.
I have seen flapper valves in half-mask
air-purifying respirators that had hair or bits
of paper towel from cleaning that caused them to
leak. Some were missing completely or
stuck open having been sucked behind their
spider support from heavy breathing. Most
of the flapper valves I have seen in disposable
N95 FFR are very thin and not very robust but
are well protected behind a non-removable
housing (cover) and not intended to be serviced.
There are much higher quality outlet valve
assemblies in elastomeric half-mask respirators
which are can be serviced. These types I
believe would be more suitable for a reusable
FFR. However, in my mind none would lend
themselves particularly well to washing,
assuming that was the primary method used to
decontaminate/reuse the masks, unless the entire
assembly or flapper valve could be removed
easily before washing the facepiece covering
and/or filter (if separate) and reinserted
without compromising the seal of the valve
assembly and/or the flapper valve.
In summary, I see the benefit of exhalation
valves in “single or limited use (i.e.,
disposable) ” N95 FFRs but due to the concerns
mentioned above not so much for “long-term or
extended use (i.e., reusable) ” FFRs, at least
those that would need to be cleaned (washed) and
maintained.
I would like to summarize my opinion in the
positive: I believe exhalation valves
would be a beneficial and desirable feature for
a “reusable” N95-equivalent FFR intended for
extended use by the general population.
That is, if the mask can be designed to be
maintained by the user (properly cleaned)
without compromising the performance of the
mask, especially with regards to the exhalation
valve.
ExxonMobil has New
Filtration Fabric and Mask Design
In response to the unprecedented challenges from
the COVID-19 pandemic, ExxonMobil is applying
its deep knowledge and experience with
polymer-based technologies in combination with
GCMI to facilitate development and expedite
third-party production of innovative safety
equipment that can be sterilized and worn
multiple times.
A
new industrial-style mask is being fast-tracked
for production. The design improves coverage of
a health care worker’s nose and mouth and will
use a replaceable cartridge system that includes
a filtration fabric to prevent contact spread of
the virus from the saturated filter. In this
design, the filters are disposable while the
main component of the mask can withstand
repeated sterilization, thus prolonging the
life-cycle of the product and addressing
shortages of N95 masks.
Prototypes are currently being tested and
reviewed by the U.S. Food and Drug
Administration. When approved, production will
begin immediately, with ExxonMobil supporting
the identification of manufacturers familiar
with the materials and process to quickly
deliver the masks to doctors, nurses and health
care providers. Once approved, manufacturers
indicate they will be able to produce as many as
40,000 ready-to-use masks and filter cartridges
per hour.
"Expediting advanced technologies to help those
who are combatting this global pandemic is
absolutely critical for society," said Karen
McKee, president of ExxonMobil Chemical Company.
"We’re proud to do our part by sharing our
expertise and experience in material
technologies, and energy supplies needed to
support our health care workers. It’s just one
example of ExxonMobil employees working around
the clock to help keep our communities safe and
limiting the spread of COVID-19."
"Scaling solutions rapidly to address the global
crisis requires significant investment,
innovation and collaboration," said Tiffany
Wilson, CEO of Global Center for Medical
Innovation. "By partnering with ExxonMobil,
we’re harnessing the expertise and capabilities
of the world’s largest energy companies to
accelerate our ability to realize that vision."
Another product developed by GCMI is a face
shield made from high-grade polymers that can
withstand the harsh conditions of sterilization
to enable reuse, while meeting the visibility
and safety requirements of current designs. The
technology complies with existing safety
standards, reducing the time from design to
front-line use. More than 50,000 units have
already been produced and are being distributed
to hospitals in New York and Atlanta. Production
facilities are ramping up to manufacture more
than 170,000 shields per hour in the coming
days.
GCMI verifies, validates and accelerates the
development and commercialization of new medical
technologies that save lives and improve patient
care. GCMI has worked collaboratively during
COVID-19 to design, develop, prototype, validate
and execute the need for protection to frontline
healthcare workers, with an efficient, quick
process intended to save lives.
ExxonMobil, which invented filtration fabric
technology in the 1960s, is making its experts
available to provide technical expertise and
delivering polypropylene from its manufacturing
sites in Baytown, Texas and Baton Rouge,
Louisiana. The raw materials will be expedited,
if needed, for face mask assembly. The company
will also facilitate supply chain interfaces to
expedite deployment.
The initiative is a collaboration between GCMI;
Dr. Joanna Newton, Pediatric
Hematologist/Oncologist, Aflac Center and Blood
Disorders Center of Children’s Healthcare of
Atlanta and Assistant Professor of Pediatrics,
Emory University School of Medicine; Children’s
Healthcare of Atlanta Pediatric Technology
Center; and a team of scientists and researchers
at the Georgia Institute of Technology and its
Invention Studio.
Justin Sink, a digital transformation and
innovation advisor at ExxonMobil, answered
questions about the masks.
The masks are made
of a material that Exxon invented in the 1970s.
It looks and feels like rigid cloth, but it’s
actually a melt-blown polymer that enables air
to flow in and out easily. What most people
don’t know is that before the fabric is shaped
into masks, it’s given an electrostatic charge.
That charge is what captures viruses or
bacteria.
Over time and with extended use and
contamination, that charge diminishes and, along
with it, the protective ability of the mask. So,
these masks often have to be thrown away after a
single use. And the problem is there’s only so
much of the fabric being produced around the
world.
With the N95 mask, the technology that’s
required to produce each unit is quite complex.
It’s difficult to build a new supply chain for
N95 masks in a week – it usually takes months.
And the virus spreads exponentially.
For context, says Sink “ we still produce the
raw materials for the fabric at our Baytown,
Texas, production facility, which is currently
working at maximum capacity. But most of the N95
microfiber is manufactured in Asia, and they
need masks there as much as we need them here.
So, the supply chain is stretched too thin,
meaning mask material that’s made in Asia is
staying in Asia right now.
Fortunately, ExxonMobil understands materials
science, manufacturing and supply chains, so
we’re working to use that expertise and our
connections in these areas to help. That means
looking at similar designs and materials and
talking to academics and manufacturers who can
divert their resources and retool their
machinery quickly.
“In
terms of materials for the masks, we have two
new sources. One is from a company that makes
the dust-repelling fabric for speakers for sound
systems. That material, when charged, gets to a
similar level of protection as an N95 mask and,
if used properly, can protect a health care
worker for an entire shift. A team at North
Carolina State University, which is the premier
fabric institute for polymers, has a production
line operating 10 hours per day. We’re helping
to get them going 24/7.
Of course, there’s only so much filtering
material that can be produced. So, we need to
use every centimeter of fabric effectively,
which means rethinking the mask itself.
So instead of the traditional N95 disposable
mask, we’re working on a composite rubber
facemask with small, replaceable filter
cartridges. This mask looks like the ones
painters wear. The filter fabric in the
cartridges will have the electrostatic charge,
and at the end of a shift, the doctor or nurse
can simply remove the mask, clean it and replace
the cartridges with new ones, enabling them to
use the same mask again the next day.
Would the masks’
replaceable filter cartridges contain similar
material to that of an N95 mask?
Essentially, yes. The main advantage is that we
will use far less filter material than we would
with a disposable mask, so no filter material
goes to waste.
Best of all, we’re not putting health care
workers in a position where they feel they have
to wash or heat sterilize an N95 mask, which
just reduces the effectiveness of the masks
they’ve been forced to reuse up to this point.
So how do we get from concept to rapid
production, given the current supply chain
constraints?
We used 3D printing for prototypes and
molds. After working with the medical community
and the team at the Global
Center for Medical Innovation in
Atlanta to make sure the mask is up to
standards, our medical-grade plastics experts
started working with private and government
manufacturers to create an injection mold.
Once the mold is built in the shape of the mask,
thousands of them can be produced per hour.
We’re collaborating with NASA, the Georgia
Tech Research Institute,
GE, Delta Airlines, the National Organization
for Technology Exploration and Delivery, Boeing
and the U.S.
Army
Spectrashield Meets Efficiency Requirements
The filtration technology in the SpectraShield™
Series of antimicrobial respirator masks
formally passed penetration and resistance in
multiple testing at numerous independent testing
laboratories in the European Union. These tests
require the SpectraShield™ masks to be subject
to exposure of a quantity of particulate
aerosols at .3 micron in size at a specific
velocity rate. Upon the exposure of the
aerosols, the amount of droplets that penetrate
the mask are measured. In the European Union,
for the masks to be rated a FFP2 it must meet a
minimum of a 97% filtration rate, and for a
FFP3, it must meet a minimum 99% filtration
rate.
In addition to conventional testing for a
disposable respirator mask, the SpectraShield™
mask was also subjected to rigorous testing for
reusability in which each mask was tested for
filtration performance, inhalation and
exhalation minimum tolerances after the masks
had been subjected to severe clogging in a
dolomite dust test. Two of the SpectraShield
masks passed this rigorous reusability testing
to earn a classification of FFP2 RD and FFP3 RD.
The tests
conducted at these various independent
laboratories were conducted according to
national and international testing standard for
filtration and safety. All the independent
testing laboratories used in testing the
SpectraShield™ masks are recognized by the
applicable regulatory agencies in the European
Union.
Extensive toxicology testing has been performed
by AgION regarding the silver-copper zeolite
antimicrobial agent. Independent tests results
indicate the antimicrobial agent to be safe and
non-toxic causing no negative side effects,
conditions, or consequences.
Berry Global has New Material for Surgical Masks
Berry Global will
increase production of face mask materials. The
initiatives include additional capacity for the
production of face mask materials in North
America and a new material for face masks in
Europe. With demand outpacing current supply for
face mask filter media, the product development
team at Berry has responded to deliver
innovative solutions in a matter of weeks to
support the demand. These solutions include
pivoting existing manufacturing assets and
creating alternative materials for face masks.
Berry has
expanded its proprietary Meltex platform to add
meltblown capacity in Waynesboro, VA. The line
will make meltblown materials which will
ultimately be used in surgical-grade face masks
along with N95 and N99 respirators. This added
capacity will support the manufacturing of
approximately 200 million face masks annually.
Berry is also launching an extension to its
Synergex range of products, Synergex ONE, a new
media for face mask applications. Developed to
initially meet the new face mask categories for
general population, the aim is to quickly bring
the media up to EN 14683:2019 standards for
surgical masks. The newly introduced Synergex
ONE provides a multilayer nonwoven composite
product in a single sheet, as an alternative to
traditional face mask layer structures.
This new material will be manufactured in Europe
and serve the European market and is available
immediately.
“This was
something that was of paramount importance in
the short term development,” says Cedric Ballay
EVP & GM for Europe in Health, Hygiene, and
Specialties for Berry. “Given the array of
materials currently being offered to the market,
we are proud to offer an alternative solution to
the traditional charged meltblown. We are now
continuing to push on with the development to be
able to pass BFE Type I and Type II testing with
this media.”

Features are
·
Multi-layer composite material – no lamination
needed
·
Filtration core of unique meltblown technology
·
Suitable for general use
NC State Develops
Two Polymer Spunbond for Masks Which Can Be Sewn
and is Washable
NC State’s Nonwovens Institute (NWI) is using
its two research and training pilot production
lines to produce face mask materials that will
be used to protect medical workers on the front
lines of fighting the effects of COVID-19.
Surgical face masks are made with nonwoven
materials, says Behnam Pourdeyhimi, executive
director of NWI, Wilson College of Textiles
associate dean for industry research and
extension and William A. Klopman Distinguished
Professor.
Because of the current critical need for masks
caused by COVID-19, Pourdeyhimi and his NWI team
created a new spunbond material that can serve
as an effective filter without the need for a
meltblown filtration layer. The unique fabric is
composed of two different polymer materials that
are combined to make a single fiber with
significant strength and bulk – and that shows
effectiveness in filtration similar to current
materials used.
“Because of the COVID-19 crisis, we took the
spunbond technology and created a new generation
of unique filters that have excellent filtering
capability and can potentially be reused after
cleaning with peroxide, or potentially alcohol
solution,” Pourdeyhimi said. “Because these
materials are strong, unlike classical meltblown
filters, they can also be cut and sewn by
traditional techniques.”
Typically, one meter of spunbond material
provides enough material for about 20 to 25
masks when using the current designs,
Pourdeyhimi said. One of the NWI’s production
lines started producing 2,000 meters of spunbond
material per hour, with the potential to create
some 20,000 meters of spunbond material in a
day. NWI currently has an agreement to provide
large amounts of spunbond nonwoven material to
Brooks Brothers, which will make masks at its
manufacturing facilities.
NWI’s other production line is a
state-of-the-art meltblowing pilot line that
will make the classical meltblown material for
N95 masks and surgical masks.
“We created a recipe for the production of
classical N95 respirator materials and will ship
those materials out for industrial partners to
convert these into respirators,” Pourdeyhimi
said.
The meltblown material takes a bit more time to
produce; Pourdeyhimi estimates that his
production line can make about 12,000 meters of
material in one work shift.
Thanks to support from across the university,
Pourdeyhimi says that NC State has ordered
machines that will allow the NWI to make
surgical masks in its Centennial Campus
facilities. Those machines should arrive in the
next month.
https://news.ncsu.edu/2020/04/a-necessary-filter/
Hospital Using MSA
Respirators with Replaceable Cartridge
Health care workers in the Allegheny Health
Network have started using protective gear that
is expected to replace thousands of N95 masks.
AHN has partnered with Pittsburgh-based company
MSA Safety to secure a shipment of P100
industrial-grade respirators, the health network
announced Thursday.
The
masks are reusable and can be disinfected. When
the coronavirus subsides, the masks can be
stored and used again if needed, officials said.
They will be used by intensive care unit and
emergency department staff, as well as
caregivers working with patients who are
confirmed or suspected to have the coronavirus,
throughout the AHN system.
The MSA Advantage 200 LS Respirator style was
selected by AHN staff for its fit and comfort,
said Dr. Sri Chalikonda, AHN’s chief medical
operations officer.
Employees who are currently using multiple N95
masks per day will be prioritized, he said.
The initial shipment includes 4,000 masks.

The P100 masks, which are not typically used in
health care settings but are approved for
industrial use by the National Institute for
Occupational Safety and Health (NIOSH), cover a
person’s nose and mouth, and are equipped with
two removable filter cartridges.
They will be sterilized between uses.
“MSA recognizes that fighting the spread of
COVID-19 requires an all-hands-on-deck
approach,” said Steve Blanco, President of MSA’s
Americas business segment. “We are pleased to be
working alongside AHN and other leading health
care providers to explore and deliver PPE
solutions that are helping communities better
respond to this unprecedented challenge.”
The U.S. Food and Drug Administration and the
Centers for Disease Control and Prevention
approved the use of such respirators in health
care settings during the coronavirus pandemic in
early March.
MSA Has a Range of
Respirator Designs Available to Protect Hospital
Personnel
The CDC has specified that
reusable National Institute for Occupational
Safety and Health (NIOSH)-approved elastomeric
respirators are a viable option for use by
healthcare workers and the first responder
community. This document outlines MSA Safety’s
NIOSH approved respirators and filter
configurations that meet CDC’s guidance. Two key
considerations when selecting an air-purifying
respirator (APR) for protection against COVID-19
are respirator type and protection level. While
there are many types of respiratory protection,
CDC recommends NIOSH-approved masks with a
protection factor of N95 or higher for certain
healthcare workers who may be exposed to
COVID-19. In the link MSA provides a comparison
of respirator types, including supplied-air
respirators; filter classifications regulated by
NIOSH that meet CDC recommendations;
Sindat Now Producing Efficient Masks with
Replaceable Membrane
This major Czech company has developed a
comfortable cotton mask with a replaceable
membrane with high virus capture efficiency. The
mask is washable. A package consists of a mask
and 10 membrane inserts at a price around 10
Euros. It is recommended that the membranes be
worn only once but McIlvaine contacted company
officials who agreed that with a day of limited
use the membrane could be used a second day.
The cost per wearing could therefore be
well below 1 euro.
Cummins and Dupont are Working Together to Help
Address the Current Shortage of N95 Masks
The need for masks has skyrocketed in recent
weeks due to the global pandemic, and Cummins
will use its NanoNet® Media to help answer that
need.
According to Amy Davis, Vice President of
Cummins Filtration, with many of the world’s
leading mask manufacturers in need of the
critical materials to assemble the masks
and struggling to meet demand, Cummins will
use pre-existing filter technology in
partnership with DuPont to help fill the supply
void.
"Cummins is re-evaluating our supply base and
manufacturing capabilities to identify how we
can support our healthcare professionals who
rely on critical personal protective equipment
to do their jobs," Davis said. "Our NanoNet®
Media can fill a key supply void and help
address the mask shortage facing the United
States and other countries around the world."
The project also aims to provide open source
instructions that other healthcare systems and
groups can use to create their own respirator
masks.
Cummins’ NanoNet® and NanoForce® Media
technology, which uses DuPont’s Hybrid Membrane
Technology (HMT), can typically be found in air,
fuel and lube filtration products used in
heavy-duty diesel engines to prevent long-term
engine wear, but also can be used in the N95
respirator masks worn by healthcare
professionals to filter harmful airborne
particles that can spread COVID-19.
The N95 designation means the respirator can
block at least 95 percent of particles from
entering the wearer’s nose and mouth. When
Cummins’ NanoNet® Media was tested using an
industry standard testing method, it exceeded
the performance requirements for N95
designation. Cummins’ manufacturing facilities
have since provided media samples to mask
manufacturers across the globe to test its
effectiveness.
![]()
Stock of Cummins Filtration NanoNet® and
NanoForce® Media technology winding.
While products featuring Cummins’ media will
need to be vetted and approved by the National
Institute for Occupational Safety and Health
(NIOSH), the company is preparing to do its part
to help relieve the burden facing the healthcare
industry.
“We’re working as quickly as possible with
healthcare regulators and other partners to help
certify products with our materials, and prepare
our manufacturing facilities to meet demand,”
added Davis.
The first mask prototypes using Cummins’ donated
media were assembled by University of Minnesota
teams in March as part of an initiative to
provide masks to M Health Fairview and other
Minneapolis-based healthcare systems. As the
COVID-19 outbreak escalated, the University of
Minnesota realized their supply of N95 masks to
protect healthcare workers would potentially run
out in a matter of weeks.
![]()
To address this challenge, a
team of designers, engineers,
chemists, surgeons,
anesthesiologist and apparel and
clothing experts from the
University of Minnesota’s
Institute for Engineering in
Medicine; Medical School;
College of Design; College of
Science and Engineering; and
Center for Filtration Research
Consortium (CFR) came together
to address this projected
shortage of critical personal
protective equipment.
Advanced, high-performance media for N95
respirator manufacturing.
"The first thing we recognized from our experts
in the Center for Filtration Research, who work
directly with Cummins, is that not all
filtration materials are created equal and that
the Cummins material is an excellent
alternative," said Jakub Tolar, Campus Health
Officer and Medical School Dean at the
University of Minnesota.
"We are tremendously grateful for the generous
donation from Cummins of their filtration
materials toward our mask effort. Since the
arrival of the filtration media, we have been
able to make rapid progress, and we now believe
we have several viable mask options, including
both a disposable and re-usable option. These
designs show real promise in keeping our
healthcare workers safe should standard medical
supplies of N95 masks no longer be available,”
continued Tolar.
While DuPont’s innovative and unique Hybrid
Membrane Technology (HMT) is typically
integrated with Cummins’ synthetic fibers to
protect sensitive engine components, it has
multiple other applications that can include
filtration media used in N95 respirator masks.
DuPont’s Hybrid Membrane Technology goes beyond
the limits of traditional semi-porous or
nonwoven membranes for air and liquid
filtration. Made using a proprietary spinning
process, the hybrid technology materials are
comprised of continuous sub-micron fibers. The
end result is a “membrane-like” sheet structure
that balances breathability and high filtration
efficiency of particulates.
“We are proud to make our advanced technology
available to help protect more caregivers on the
front lines of this global health crisis,” said
HP Nanda, Global Vice President & General
Manager, DuPont Water Solutions.
“We thank our partner Cummins for transitioning
the use of its production line to help address
the global shortage of N95 mask materials, and
we thank the experts at the University of
Minnesota for their leadership in testing and
designing several mask options for the benefit
of many healthcare systems," Nanda added. "By
working together—and innovating new applications
of existing technologies and materials—we hope
to slow the spread of this terrible virus."
U.S Army Research on Face Mask Media will be
Helpful
The U.S. Army Edgewood Chemical Biological
Center (ECBC) Respiratory Protection Branch
members investigated novel aerosol filtration
materials for inclusion in the next generation
respirator. Commercial particulate filtration
technologies with high-efficiency and
low-pressure drop have the potential to provide
improved protection to the Warfighter while
decreasing breathing resistance and thus
reducing physiological burden.
A pressure drop of ≤5 mmH2O was selected as the
goal for the development of next generation
lower burden filters.
The aerosol filtration penetration requirement
for the M61 filter is ≤0.01% (i.e., 99.99%
efficiency) when measured at a constant flow
rate of 25 L/min (equivalent to 50 L/min through
the pair of filters). Each filter has an
effective airflow area of approximately 60 cm2 ,
which results in a face velocity of
approximately 7 cm/s when measured at 25 L/min.
The particulate filter element of the M61 filter
consists of pleated HEPA media and is roughly 6
mm thick. The market survey was limited to media
with the potential of achieving efficiencies
≥99.97% (HEPA quality).
While this target is below the JSGPM
requirement, efficiencies of 99.99% can be
achieved through pleating the media, which
reduces the face velocity and increases the
collection efficiency of the filter. This
reduction in face velocity increases the
collection efficiency of the filter. In the case
of flat sheet electrets (nonwoven electrostatic
charged media), the thickness can be increased
to meet HEPA requirements. Efficiency can be
improved by other means to maximize the
effective surface area, for example, by using
larger and more efficient filter designs similar
to those being considered for future integrated
respirator/helmet systems.
To avoid eliminating promising media, the market
survey did not take into consideration the
thickness of the media; however, a total
effective surface area of 250 cm2 was used as
the basis for the 5 mmH2O pressure-drop goal to
take into account the increased surface area
realized by the emerging advanced filter
designs. Taking these goals into consideration,
a market survey was conducted to identify new
HEPA quality filtration media with equivalent or
greater capture efficiency and lower pressure
drop than the particulate media currently used
in military air-purifying respirator filters.
Only commercial manufacturers were considered.
Here are the conclusions.
Fibertex Non Wovens has New HEPA Filter Media
for Filters and Respirators
Fibertex Nonwovens has
introduced a new fully synthetic non charged
HEPA 13 filter media based purely on mechanical
filtration by nanofibers and with near to half
the pressure drop of glass media.
Designed for use in various applications,
including vacuum cleaners, air purifiers, HVAC
systems and respirators, Pleatex 80AH13NP6 is
made from durable non-shredding nonwovens, which
can replace hazardous glass fibres that are a
risk when processing or when replacing filters
in HVAC systems. Fibertex Pleatex 80AH13NP6 is
easy to process on all types of pleating
machines, including rotary pleaters, knife and
blade pleaters. Advantages of this 100%
synthetic material over commercially available
glass fibre products include faster pleating, a
low pressure drop and long-term efficiency. This
material also adheres to the lowest energy
consumption standards in ventilation systems.
Fibertex Pleatex 80AH13NP6 is based purely on
mechanical filtration by nanofibres which are
non-charged and is most commonly available as an
80 gsm HEPA 13 pleatable filter media. The
benefit is that Fibertex Pleatex 80AH13NP6 works
efficiently in all conditions and has a longer
shelf life compared to charged melt-blown media,
Fibertex Nonwovens using in-house
state-of-the-art nano technology to produce its
new highly efficient filter media and is
manufactured to the highest quality in Aalborg,
Denmark.
The company has
introduced a new fully synthetic non charged
HEPA 13 filter media based purely on mechanical
filtration by nanofibres and with near to half
the pressure drop of glass media.
Designed
for use in various applications, including
vacuum cleaners, air purifiers, HVAC systems and
respirators. Fibertex Pleatex 80AH13NP6 is easy
to process on all types of pleating machines,
including rotary pleaters, knife and blade
pleaters. Advantages of this 100% synthetic
material over commercially available glass fibre
products include faster pleating, a low pressure
drop and long-term efficiency. This material
also adheres to the lowest energy consumption
standards in ventilation systems.
The
media is based purely on mechanical filtration
by nanofibres which are non-charged and is most
commonly available as an 80 gsm HEPA 13
pleatable filter media. The benefit is that
Fibertex Pleatex 80AH13NP6 works efficiently in
all conditions and has a longer shelf life
compared to charged melt-blown media,
Fibertex Nonwovens using in-house
state-of-the-art nano technology to produce its
new highly efficient filter media and is
manufactured to the highest quality in Aalborg,
Denmark.
We see a huge potential in the filtration
market, and our ambition is to become a leading
global player capable of manufacturing filters
with a wide range of different properties. In
addition, we have the facilities to produce
these products under fully controlled and
certified production conditions ensuring
environmentally-responsible and sustainable
production,” said CEO,
Jørgen Bech Madsen
“By
utilizing premium, high-efficiency Fibertex
nanofibre layers in the production of protective
face masks, end-users can expect to reach N95
and FFP2 level of protection," says Per Holst
Rasmussen of Fibertex Nonwovens. “The main
benefit of Fibertex nanofibre layers, compared
to electret meltblowns currently used in masks,
is guaranteed efficiency of the filtration layer
during the lifetime of the mask. Fibertex
nanofibre layers are based purely on mechanical
filtration, unlike electret meltblown materials,
which tend to become discharged during usage and
substantially lose filtration efficiency,
especially in conditions of over 80 % relative
humidity.
“Another
important benefit of this advanced technology,
is durability of the nanofibre layer, which
enables extended storage time of masks, making
them suitable for use as disaster and epidemic
relief safety stocks.”
Alternative coverstock media from
Ahlstrom-Munskjo
“The medical business unit’s average coverstock
fabric production contributes to an estimated
50-60 million finished face masks per month. As
a result of the increased demand from the
COVID-19 pandemic, that number has nearly
tripled. Ahlstrom-Munksjö is now producing the
coverstock fabric being used to make over 150
million face masks per month,” commented Bonte.
Ahlstrom-Munksjö's common business platform
provides global resources, which has a positive
impact on supporting customer demand in the
healthcare industry. For example, the Brignoud plant
in France, part of the Ahlstrom-Munksjö’s Nonwovens
business, has offered
an alternative outer coverstock fabric to help
meet local requests in Europe. The
company also brings additional production
capacity to the market at the Turin,
Italy plant which is part of the Filtration
business. There, they have started production
for face mask materials with a machine usually
used for industrial filtration materials.
As a global company, Ahlstrom-Munksjö is
expanding the Medical business’ reach and
staying connected on a national and
international level based on official updates
provided by local government and institutions as
well as international organizations. Across the
world, individuals and corporations are doing
the best to identify innovative solutions to
address the critical needs for protective
apparel. Subject matter experts at
Ahlstrom-Munksjö’s Medical business team are
also providing support and guidance wherever and
whenever possible.
For decades the medical business has been
making fabrics used to construct surgical gowns
and drapes, pleated surgical face
masks, protective apparel and sterile barrier
systems. These high performing medical fabrics
are used around the world in operating rooms and
clinical environments to protect health care
workers and patients against viruses, bacteria
and fluids.
Not all medical nonwovens created equal
Not all medical nonwovens are created the same
way; they have different levels of protection
and properties for breathability and comfort.
Some fabrics are highly breathable, which helps
to keep the wearer cool and comfortable but have
limited barrier protection. Some offer a high
level of barrier protection but are not
breathable. Having a technology that offers both
protection and comfort is difficult to
achieve.
Ahlstrom-Munksjö‘s next generation breathable
viral barrier (BVB) fabric offers both
protection and comfort. Its tri-laminate
fabric is constructed to be impervious,
breathable and comfortable. The outer layer is
fluid repellent and durable. The barrier layer
has a reformulated monolithic film membrane
making it impervious to liquids, viruses and
bacteria, while the chemical composition of the
film itself allows moisture vapor to pass
through it, keeping the surgical staff cool and
dry. The darker inner layer is designed
to reduce shadowing and is soft to the touch,
which makes it comfortable to wear for long
periods of time.
Ahlstrom-Munksjö’s Medical
business employs around 700 people with sales
of approximately EUR 90 million in 2019. It has
a global sales network and operates two plants
and two converting lines.
In the Medical business, Ahlstrom-Munksjö continues
to expand and enhance its medical product
portfolio with personal protective apparel
fabrics and grow market share in surgical drapes
and gowns. In sterilization wraps, the
company strives to strengthen its leading
position in Europe and grow as a solutions
provider through an enhanced service offering.
Social Distancing Alternative with Low Risk and
Modest Cost for Coronavirus Mitigation
Social distancing needs to be redefined as
staying six feet apart of other individuals or
the space they have occupied in the last 60
seconds. This expanded definition creates great
barriers to normal life. The safety of social
distancing can be alternatively provided with a
program which will have comfort and social
acceptance along with affordability. This is now
possible because of new filtration media,
testing, decontamination and other technology
innovations.
It starts with
the fact that a washable highly efficient
mask can be provided.
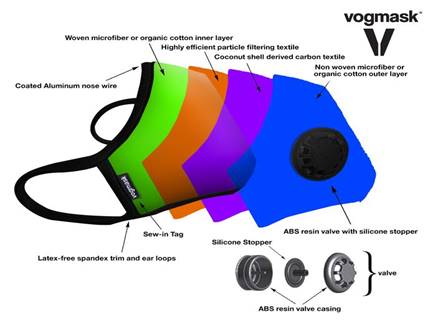
There are retail stores in China
which sell nothing but these masks with
many fashionable varieties. With the valve they
are both comfortable and stylish. They are also
washable. These are $30 masks which if worn
60 times would cost $.50 per wearing.
There are less expensive designs available as
well. Chinese companies are also producing
washable N95 masks with and without the valve
feature. Over the last few months the production
has expanded to several million per day. Since
they are washable the cost per wearing is as low
as 10 cents.
The valve feature makes the wearer comfortable
but since breath is discharged unfiltered it
should only be used by people without symptoms.
The Mask Market Could Soar From a Few Billion
Per Year to Hundreds of Billions
At a choir practice in a Washington State Church
in February precautions including sanitizing
everything and social distancing were taken
during the two hours when 60 people assembled.
Yet 45 of them contracted the coronavirus.
The conclusion was that the act of singing
created an air transmission route which was
deadly. Hundreds of Diamond Princess passengers
were not infected until they spent weeks in
their cabins breathing the air from a common
HVAC system.
When the air transmission of droplets smaller
than 5 microns is accompanied by interaction of
individuals who may not show symptoms you create
a situation where masks become very important.
It is not surprising that the Chinese death toll
per capita is very small compared to Italy. For
Chinese efficient masks have been purchased for
years.
Vogmask opened its first retail store in China
in 2013.
This U.S. based company had all the
needed testing on efficiency and resistance done
by Nelson laboratories.

These stores have the aura of a sportswear store
elsewhere in the world. The masks sell for $33
but can be washed 20 or more times.
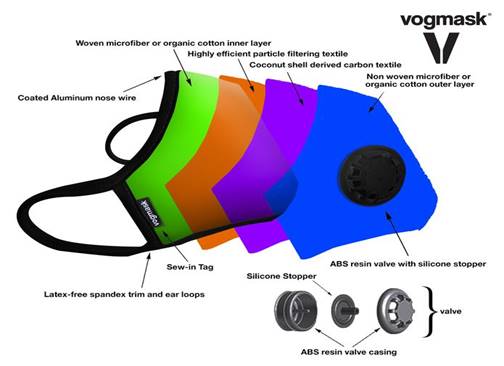
If just ten percent of the world’s population
wore these masks that would be 800 million
wearers
With 20 wearings the cost per wearing
would be $1.50.
This alone would create a $1.2 billion
per day market or $438 billion per year.
But the poorer people could also be wearing
effective masks. China is making N95 quality
masks with a nanofiber laminate on a cotton
backing.
The masks can be washed at least 20 times
at a cost per day of just 15 cents.

If 2 billion people were wearing these masks the
cost would be $300 million per day. Or $109
billion per year.
This would create a market of over $500
billion per year for mask makers. Based on the
cost of a life lost at $50 million this expense
can be justified if just 10,000 lives are saved
per year.
The question for media and mask makers is how
long will this boom last? The answer is very
likely 36 months and very possibly permanently.
It is expected that the southern hemisphere will
experience an epidemic during the northern
summer. Then infected individuals will travel
North and there will be a spike in the winter of
2020-21.
The practice of wearing masks is likely to be
permanent for the following reasons
-
Fear of another pandemic with a
new virus
-
Realization that there are
millions of cases of flu each
year and some can be prevented
-
The emotional phenomena
which could cause an
exaggerated view of the risk
similar to fear of being eaten
by a shark if swimming in the
ocean.
-
Demonstration of compassion –
not wanting to infect anyone
with anything
The nanofiber laminates create all sorts of
possibilities. There are also new designs
available which provide a permanent mask with
replaceable media.
At least one Chinese company is offering
this design. Exxon Mobil just announced a design
which provides improved coverage of
the nose and mouth in a structure which
can withstand repeated sterilization. A
replaceable cartridge with N95 efficiency is
utilized.
Exxon Mobil is waiting for FDA approval and then
hopes to produce 40,000 masks and cartridges per
hour.

Superior Felt and Filtration has Both Meltblown
and Needle Punched Media for Masks
Superior Felt & Filtration supplies a wide array
of nonwoven synthetic fabrics for the
manufacturing of safety & personal protection
filters. It is among the top nonwoven synthetic
filter media suppliers for medical and emergency
response textiles, such as respirators and
masks. It is one of the largest manufacturers of
micron and sub-micron filter media for
respirator and medical applications in the U.S.
The company offers non wovens that can be
utilized in masks, air purifiers, medical
equipment, personal safety apparel and
cleanrooms that are highly efficient against 0.1
micron particles. The electrostatically charged
high alpha perm melt blown and needle punched
products can be easily molded into masks,
pleated and die cut to offer protection over
99.9% against 0.1 micron particles which are
considered to be the most penetrating particle
sizes (MPPS). The electrostatic media offers low
air flow resistance for more breathable masks or
devices that help reduce fatigue & improve
comfort levels.
Technostat® can also be utilized with breathable
laminates, activated carbon and other materials
for combined dust and gas filtration. For these
reasons, Technostat® is ideal for nonwoven
synthetic filter media for respiratory
applications. In addition to Technostat® filter
media, the company also offers
Technostat® Plus – a triboelectric media of
needle-punched felt that offers 20% improvement
in filtration efficiency over standard
electrostatic filter media. This nonwoven
synthetic fabric produces its triboelectric
properties when 2 dissimilar fibers used during
the manufacturing process create a charge that
enhances filtration capabilities.
Superior Felt & Filtration also provides
electrostatic filter media rolls (electrostatically-charged
synthetic needle punch fibers) and melt blown
fibers. These nonwoven synthetic fabrics aid in
producing some of the highest levels of
filtration for health care providers and
emergency responders.
SWM supplies Melt Blown Media and Film for
Surface Layer
SWM International says it stands ready to
supply converters and manufacturers of face
masks with advanced nonwoven materials necessary
to meet the global challenge presented by the
coronavirus outbreak.
“SWM has a long history of supplying
high-quality media integral to the construction
and performance of face masks used in the dental
and surgical sectors as well as the industrial
sector,” said Bart Sistrunk, SWM’s Commercial
Director – Filtration. “Our DelporeTM meltblown
media is widely used in face masks because it
provides excellent breathability without
sacrificing Bacterial Filtration Efficiency
(BFE) and its lightweight nature allows for
comfortable wear.”
A leading producer of meltblown media, SWM also
offers
DelnetTM apertured
film, a lightweight nonwoven that is extruded,
oriented, and uniquely embossed for use as a
flexible surface layer for medical facemasks
or as a comfort barrier in finger bandages.
“SWM is prepared to prioritize production of
Delpore meltblown media and Delnet apertured
film for customers who need materials for face
mask production,” said Sistrunk. “We are
committed to continued support during the
current world health emergency.”




