
Coronavirus Pharmaceutical Solutions
June 3, 2020
·
Coronavirus vaccine would
create a market of up to $ 40 billion/yr
·
Single use closed systems can shorten the
timeline to a successful COVID vaccine.
·
Sanofi has New Closed System with Single-Use
Technologies:
·
In house manufacturing helping speedy
progression of mRNA COVID vaccine, says Moderna
·
Deciding on spare production capacity is
difficult
·
Will modular cleanroom design accelerate therapy
and vaccine production to fight COVID
·
Stringent environmental controls needed in
vaccine manufacturing
·
Do Moderna executives have
faith in their product?
·
Fauci defends comments relative to a vaccine
within 18 months
·
Challenge to prioritize COVID over other
clinical trials
·
“May be effective” is not the criterion for
widespread vaccine use
·
Pfizer says vaccine likely
available by end of October
____________________________________________________________
Coronavirus vaccine would create a market of up
to $ 40 billion/yr
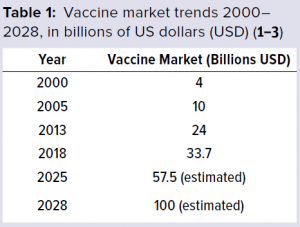
The global vaccine market has been growing
rapidly and thus has been attracting interest
and new players. The World Health Organization
(WHO)
has reported that from 2000 to 2013, the
vaccine market grew from 4 billion US dollars
(USD) to 24 billion USD (Table 1).Prior to the
coronavirus
market trends suggested that by 2028, the
value of the vaccine market was estimated to be
about 100 billion USD, growing at a compound
annual growth rate (CAGR) of 11.02%. And, more
than 120 new products are in development, 60 of
which are of special importance for developing
countries.
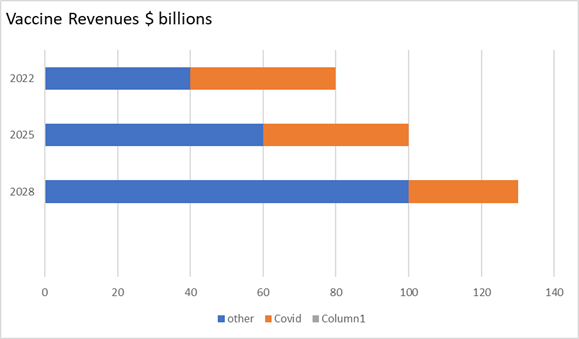
Assuming a reliable COVID vaccine is developed
for sale in 2021. Initial sales could be $40
billion dropping to $ 30 billion by 2028.
The suppliers of cleanrooms, bioreactors,
centrifuges, and other process equipment will be
challenged to provide the products and
construction services needed to meet the
requirements.
What role will closed systems, single use
systems, modular rooms, pods, RABS, isolators,
biosafety cabinet and other approaches play?
Will some be faster track options?
The mobile trailer or Pod approach offers the
fastest track but has disadvantages compared to
modular and stick built cleanrooms.
It is the most costly approach.
It is less adaptable to change mid
project.
With the variations and uncertainties
relative to COVID, this would be a problem.
There may be a need for process design
changes.
|
|
Stick built |
Modular
|
trailer |
|
Less space |
A |
A |
C |
|
Shortest time |
C |
B |
A |
|
Least cost |
A |
A |
C |
|
guarantee |
C |
A |
A |
|
Adaptable |
A |
A |
C |
|
Late change |
A |
B |
C |
|
Quality
|
C |
A |
A |
|
Predictability
|
C |
A |
A |
|
Compliance |
C |
A |
A |
|
Durability
|
C |
A |
A |
·
A most favorable B in betweenA
most favorable B in between
·
C least favorable
The markets for diagnostics and therapeutics
could be even bigger than for the vaccines.
How will this impact the availability of
products and services?
Single use closed systems can shorten the
timeline to a successful COVID vaccine.
The vaccine industry is challenged to develop
high-quality products at decreased cost and
within shortened timelines. The need to reach
the market first highlights the importance for
fast process development strategies and
techniques. Such pressures have driven the
vaccine industry to embrace innovative
technologies. In return, shortening process
development times will accelerate overall
vaccine product development timelines and
rapidly deliver safe and high-quality products
to a global market.
Some vaccine manufacturers face additional
difficulties. These include the need to work
with small batch sizes and varied product
portfolios.
Pandemic outbreaks that require rapid responses
from vaccine developers and use of highly potent
ingredients place large demands on cleaning
processes. Given such pressures, single-use
fill–finish assemblies can be an attractive
option for vaccine manufacturing facilities.
The biologics industry has been using single-use
technologies for over 10 years to the point at
which it can be considered mainstream in
bioprocessing, including in vaccine development
and manufacturing. Key advancements include
single-use bioreactors to disposable products
for final formulation and filling. A number of
vendors provide single-use bioreactors,
centrifuges, depth filters, column
chromatography systems, and bags for media
preparation and final formulation
Sanofi has New Closed System with Single-Use
Technologies:
Some single-use systems have been established in
process development and are making their way
into manufacturing. For example: Sanofi has
developed a closed, single-use process system
(from bench top to 100-L scale process) for a
serum-free, live viral vaccine product, with
close collaboration between suppliers and the
vaccine industry. In addition, staff found the
operations of the closed system much easier to
manage than the previous system. Because it
produces higher yields, the new system required
fewer production runs. And the use of disposable
technologies ensures that sterility is
maintained throughout the process.
The net result was an overall reduction in
development time and manufacturing costs.
Facility turnover was easier and faster with
this than with the previous system, and
validation of fixed assets such as stainless-
steel fermenters, tanks, and centrifuges was no
longer necessary.
Application of single-use technologies provided
opportunities to reduce fixed costs, amount of
equipment, and cleaning validation, while
increasing facility and process flexibility and
accelerating process development time.
Disposables play a key role in addressing the
industrial challenges associated with developing
high quality processes while driving down
operational costs.
In house manufacturing helping speedy
progression of mRNA COVID vaccine, says Moderna
Moderna Therapeutics believes its Norwood,
Massachusetts manufacturing plant is a
“competitive strategic advantage” in the race to
develop and scale-up a vaccine against COVID-19.
Moderna released data from a small Phase I study
of its candidate mRNA-1273 last week. The
trial, conducted
by the
National Institute of Allergy and Infectious
Diseases, showed signs that the candidate
elicits antibodies at levels comparable to those
found in the blood of dozens of patients who
have recovered from COVID-19, caused by the
novel coronavirus (SARS-CoV-2).
Now the firm says a
Phase II study enrolling hundreds of patients is
expected to begin “shortly,” and a Phase III
study enrolling thousands is planned to start in
July, making it the frontrunner in the race to
develop a vaccine against the infectious
disease.
According to CEO Stéphane Bancel, Moderna’s
manufacturing strategy – and specifically its
inhouse facility in Norwood, Massachusetts –
played a major part in the speed of development
and will help expedite the candidate’s
progression through the clinic and beyond,
helping the firm achieve its goal of producing a
billion doses a year.
“I believe it is a competitive strategic
advantage of a company, having our own
manufacturing facility from raw materials to
shipping vials to clinical trials,” he told
stakeholders during a conference call yesterday.
If Moderna had been forced to use contract
development and manufacturing organizations (CDMOs),
“we could never have been so fast,” he
continued, adding that while four or five
companies could make the mRNA for the firm,
there would have been delays in waiting for
empty slots and tech transfers, as well as
losing on Moderna’s own determination to
expedite this project.
“Being in Norwood, with our own team on
equipment, on facility, allows us to tell the
team this is very important; this one needs to
move through the system much faster than usual,
and people knew the importance of this as virus
was spreading, all determination just grew
stronger with the day.”
The 200,000 square-foot Norwood site, which
opened in July
2018, has
capacity to develop materials for preclinical
toxicology studies, Phase I and II clinical
development programs, and the ability to
manufacture, test and run fill/finish operations
for its mRNA development candidates.
Thus to scale-up mRNA-1273, Moderna has
also inked a deal with Lonza with
initial manufacturing planned to take place at
the CDMO’s site in Portsmouth, New Hampshire,
with a second phase planned at its site in Visp,
Switzerland.
“The partnership with Lonza helps to be able,
assuming a 50-microgram dose, to potentially get
to one billion dose per year,” said Bancel. The
deal will increase capacity ten-fold for the
COVID candidate, though Bancel suggested the
firm would have preferred to have done all the
production inhouse if it had the option.
Deciding on spare production
capacity is difficult
David Dodds of Dodds and
Associates has weighed in on the
wisdom of in-house production.
Any in-house production facility
is an advantage – of course. It
is obvious – and has been
throughout history – that any
degree of vertical integration
on an immediately local,
physical basis provides a large
benefit – when it is valued in
hindsight, during a crisis.
But there is no good method for
assigning a dollar-value to the
intangible benefit of having
that facility immediately
available (and so empty and
waiting at all other times –
possibly for years when there is
no need for it), or to the
equally intangible liabilities
of delay and inconvenience of
dealing with a third-party,
especially one several time
zones distant. This is
completely missing from current
methods of cost-assessment and
IRR or ROI calculations.
Dodds says we need a method of valuing high
advantage/low use assets and the avoidance of
crisis, as well as the disadvantage of
constant-but-trivial inconvenience and delay in
day-to-day operations. At present, we seem to be
unable to do this.
The intangible value of contingency,
flexibility, redundancy, and robustness have
been the casualties of the last few decades of
cost-savings
Will modular cleanroom design accelerate therapy
and vaccine production to fight COVID
This subject was addressed by Mitchell Gonzales
of AES Clean Technology in Cell & Gene

Nowhere in the product development timeline does
speed to market matter more to patients’ needs,
or to the investors who make these treatments
possible than when life-altering medicines,
therapies, and devices reach the end phase of
clinical trials.
For example, existing and new cell and gene
therapies are verging on “curative” treatments,
making speed to patients’ imperative for human
conditions inducing severe and irreparable
consequences in the absence of available
treatments. Initial cell and gene therapy
commercial products (e.g., Luxturna, Zolgensma,
Yescarta) have elevated the impact and success
of these treatments.
The article documents why modular cleanroom
systems are being increasingly chosen over a
conventional construction approach due to their
accelerated design and installation schedule
advantages, as well as their reduced job-site
safety risk during installation.
Stringent environmental controls needed in
vaccine manufacturing
Aseptic processing is principally what is
employed to ensure assurance of sterility,
because some vaccines are not filterable or
cannot be sterilized by heat, gas or radiation.²
Axenic products (sterile except for the organism
of choice in the vaccine) must use aseptic
processing as well as environmental
controls/monitoring, in-situ
cleaning/sterilization of tanks and other
equipment, closed systems where possible and
even disposable single use systems.¹
Control measures of the environment include
differential pressures, air changes,
unidirectional air, closed manufacturing
processes wherever possible, qualification of
processes such as gowning, performance of media
fills, and control of environmental bioburden.
HEPA air filters should be qualified every six
months and smoke studies should be performed for
the highest controlled areas (Grade A) every
three years.
Material and personnel flows must be well
defined with traffic starting in Grade D areas,
into Grade C, then B and finally Grade A with
gowned personnel. Specific PPE for C and D
includes masks and gloves and usually something
like bulk-sterilized jumpsuits. There must be
limits to the numbers of people allowed in the
Aseptic Processing Area (APA) at any one time,
how long they may stay in the APA and control of
interventions (inherent/routine and corrective).
Gowning performance must be qualified before
personnel are allowed into the APA and they must
show adequate aseptic technique during media
fills prior to being allowed to fill aseptic
batches of product.
Bioburden of air, people, water, and gases must
be controlled. Samples sites, types of sampling
and sampling frequency should be defined in
Standard Operating Procedures (SOPs).
Levels/limits must be defined in SOPs along with
steps to be taken if levels are exceeded (e.g.,
performance of investigations) or trends are
detected.
Disinfectants may be qualified by outside
contract labs using coupon surfaces present in
the facility or by in-situ testing. Approved
disinfectants, their use-dilution and
appropriate dwell (contact) time must be listed
in SOPs.8
Do Moderna executives have faith in their
product?
Moderna executives sold stock as shares peaked
with positive announcements.
The timing of the transactions -- coupled with
concerns from some medical experts that Moderna
overstated the significance of its Phase 1
vaccine trial -- should be investigated by
authorities, former SEC officials told CNN
Business.
"The confluence creates an appearance, which may
be inaccurate, that people were in a rush to
take advantage of an early positive trial in
what is often a long and tortured development of
a new drug," Harvey Pitt, the former chairman of
the Securities and Exchange Commission, said in
an interview.
Pitt, who led the SEC from 2001 to 2003, called
the timing of the share sales by Moderna and its
leading shareholder "highly problematic." He
suggested authorities issue subpoenas for
emails, memos and other documents that can shed
light on the share sales.
Fauci defends comments relative to a vaccine
within 18 months
Dr Anthony Fauci is optimistic about developing
a vaccine in the next 12 to 18 months but is
concerned how effective the vaccine may be. He
addressed his thoughts on this in an interview
with STAT.
“The general trend on the part of the
pharmaceutical companies, because of the
enormous investment that goes into the
development of a vaccine, is that you don’t go
to the next step until you’re fairly certain
that the step you’re in is going to be
successful. The other thing is you don’t start
manufacturing anything until you have a pretty
good idea that you have a successful efficacy
signal. That protracts out the time frame. But
what we’re doing is something that’s called
developing “at risk.
What it means is that at the same time you’re
finishing your Phase 1 trial, you’re preparing
your Phase 3 trial sites, which is very
expensive, and then you’re starting to
manufacture the vaccine even before you know it
works. All that cuts months off.
We’re now completing the Phase 1 [with the
Moderna vaccine]. The initial data look very
promising from the neutralizing antibody
standpoint. And so, they’re planning to start
the Phase 3 in the first week or so of July. Not
only with the Moderna vaccine, but also very
likely with the AstraZeneca
vaccine.
And then as we get later into the summer, we’ll
get the Johnson & Johnson in clinical trials.
You need a few months at least of having
vaccinated individuals getting exposed. So,
let’s say it’s July, August, September, October.
By November, you should have an efficacy signal.
If you do and you’re already manufacturing
doses, by December and January, if you’re lucky
and if in fact it is effective, you can have a
significant number of doses available by the end
of the year, the beginning of 2021. So, I think
it’s aspirational, but it’s certainly doable.
The only thing that’s the big unknown to me is
that, is it going to be effective? I think we
could do it within the time frame that I’ve
outlined. But there’s no guarantee that it’s
going to be effective.”
Challenge to prioritize COVID over other
clinical trials
How much impact will COVID have both near term
and long term on clinical trials for other
products?
This question was addressed in Cell &
Gene by
Angela De Martini, Dean Lockhead, Leandra
Plappert, and Elizabeth Rountree, of Charles
River Associates (CRA).
As companies learn to adapt and consider
implementing new strategies to keep clinical
trials on track, in many cases the only option
is to temporarily pause or delay development
programs. For example, Provention Bio recently
paused a Phase 3 trial in type 1 diabetes and
Iveric Bio temporarily stopped a pivotal trial
in geographic atrophy.3,4 But these
decisions are not limited to smaller companies.
Eli Lilly, Bristol-Myers Squibb, and Pfizer have
announced pauses or delays in multiple
development programs.5,6
While consensus is that the impact of COVID-19
on clinical trials will be far-reaching, our
analysis suggests that the level and duration of
this impact will be especially severe in studies
that have the following characteristics:
·
Enrolled vulnerable populations, including the
elderly, the immunocompromised, and patients
with pulmonary conditions (e.g., COPD)
·
Have primary or secondary endpoints that require
in-person visits or hospital infrastructure and
equipment (e.g., CT and PET scans) for
assessment
·
Involve indications with minor safety or quality
of life implications for patients (e.g.,
“lifestyle drugs”) and indications where a
patient’s environment has a significant impact
on therapeutic success (e.g., psychiatric, and
neurological indications)
·
Are currently in the patient recruitment stage
or are in Phase 1 with healthy volunteers
·
Have trial sites located within hospitals or
tertiary academic centers and in areas with high
COVID-19 case density, potentially reducing the
availability of staff to perform required
activities and increasing the risk of adverse
events and patient loss
·
Are evaluating immunosuppressive therapies or
therapies that require complex and prolonged
hospital visits (e.g., CAR-T cell and gene
therapies)
·
Involve smaller patient populations where there
are minimal margins of statistical power or
people living with rare diseases (because
patient accrual is already difficult, and the
pandemic increases the risk for patient loss)
While most regulatory agencies have stated an
intention to be flexible during these
unprecedented times, it is critical that drug
developers continue to monitor their risk
diligently and develop contingency plans to
adapt and respond to factors that affect their
clinical development programs. Sponsors will
need to balance patient safety, trial integrity,
and statistical power considerations against
funding and revenue issues on a trial-by-trial
basis. In addition to reevaluating clinical
development plans, companies may need to
reassess their assumptions around pricing and
revenue as well as the competitive landscape for
both their pipeline and in-line products.
There will not be a one-size-fits-all solution
for the many short- and long-term challenges
caused by COVID-19, but companies that are
willing and able to rapidly adapt are likely to
have the strongest chances for success.
https://www.cellandgene.com/doc/covid-clinical-trials-understanding-the-long-term-impact-0001
“May be effective” is not the criterion for
widespread vaccine use
Steven Joffe and Holly Fernandez Lynch of the
University of Pennsylvania Perelman School of
Medicine addressed the wisdom of accelerated
vaccine use in a Washington post editorial.
The potential benefits of emergency
authorization for a therapeutic drug are clear:
seriously ill patients get access to a therapy
that might help them. But there are downsides.
The drug might be unsafe or ineffective.
Availability of the drug also might divert
patients from trying more promising alternatives
or participating
in clinical trials needed
to demonstrate what actually works.
The trade-offs are very different when
considering emergency authorization for products
intended to prevent rather than treat disease.
The FDA has used this pathway for a vaccine only
once, in 2005, allowing a decades-old vaccine to
be used to prevent anthrax in
a bioweapon attack.
When the agency authorized emergency use of
other preventive drugs, as it did for anthrax in
2008 and H1N1 influenza
in 2009, the drugs were already FDA-approved.
Emergency authorization of a new vaccine for the
novel coronavirus would be unprecedented.
Emergency treatments may be given to thousands,
or tens of thousands, of very sick patients. But
a vaccine could reach millions — even tens of
millions or hundreds of millions — of healthy
people. Because vaccine recipients are not sick,
their potential to benefit stands to be much
less than that of seriously ill patients given a
drug, especially in light of the effectiveness
of alternatives such as physical distancing and
good hygiene. If data ultimately shows that
vaccine effectiveness is limited while the risks
are substantial, harms to millions of uninfected
individuals could
be enormous.
False reassurance from a flawed vaccine might
also worsen the epidemic, setting back progress.
Public trust in
vaccines is another critical consideration.
Already, the anti-vaccine movement has begun to sow
doubt about
a coronavirus vaccine. Even if an option
ultimately proves to be safe and effective, it
won’t do much good if many people refuse
it.
And if a covid-19 vaccine causes harm, efforts
to counter other vaccine-preventable diseases
may be set back as well.
For all these reasons, the FDA must
apply rigorous standards when
considering whether to authorize a coronavirus
vaccine. The agency must wait until data from a
well-controlled trial demonstrates that a
vaccine is able to prevent covid-19
and that it is safe enough to give to millions
of people.
There is acute need for a vaccine that will
change the course of this pandemic and allow
normal economic and social activity to resume.
Experience suggests that the FDA will be under
intense pressure to authorize emergency use of
the first plausible candidate, even if data
supporting its benefits are thin. But when it
comes to vaccines, “may be effective” is not
good enough.
https://www.washingtonpost.com/opinions/2020/06/02/fda-should-not-rush-covid-19-vaccine/
Pfizer says vaccine likely available by end of
October
Pfizer
CEO, Albert Bourla, believes they will have a
COVID-19 vaccine ready by the end of October.
The company is currently in the clinical trial
stage of
development, crafting their vaccine alongside
Germany's BioNtech. The companies, whose project
relies on messenger RNA technology never used in
an approved vaccine, dosed the first humans in
Germany earlier on in May, and hope to begin a
US trial soon, pending regulators' blessing.
If things go well, and the stars are aligned, we
will have enough evidence of safety and efficacy
so that we can... have a vaccine around the end
of October," said Bourla, according to AFP.
Pfizer, BioNtech and other companies are racing
to develop a vaccine, since there are currently
no approved treatments and only mixed results of
medicines under study against the virus.
Pfizer aims to make 10-20 million doses of the coronavirus
vaccine by
the end of 2020 for emergency use should it pass
tests, the US drug maker's head of vaccines
announced in May.
